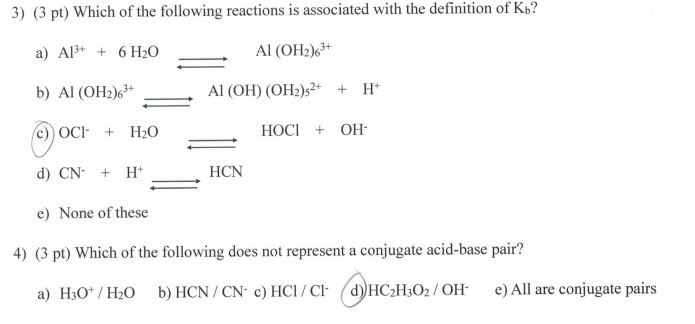
SOLVED: Predict the products of each acid-base combination listed. Assume that a neutralization reaction occurs. HBr and Fe(OH)3 HNO2 and Al(OH)3 HClO3 and Mg(OH)2
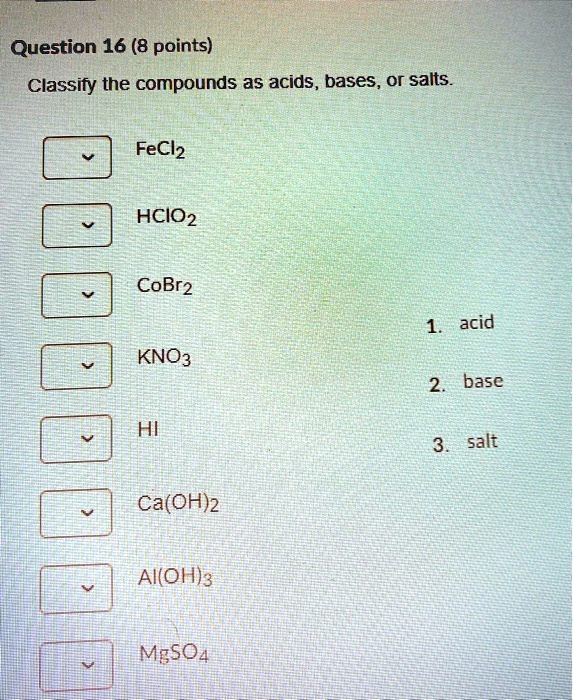
SOLVED: Question 16 (8 points) Classify the compounds as acids, bases, or salts FeCl2 HCIO2 acid KNO3 basc HI salt ca(OH)2 AICOH)3 M,5o4 CoBr2
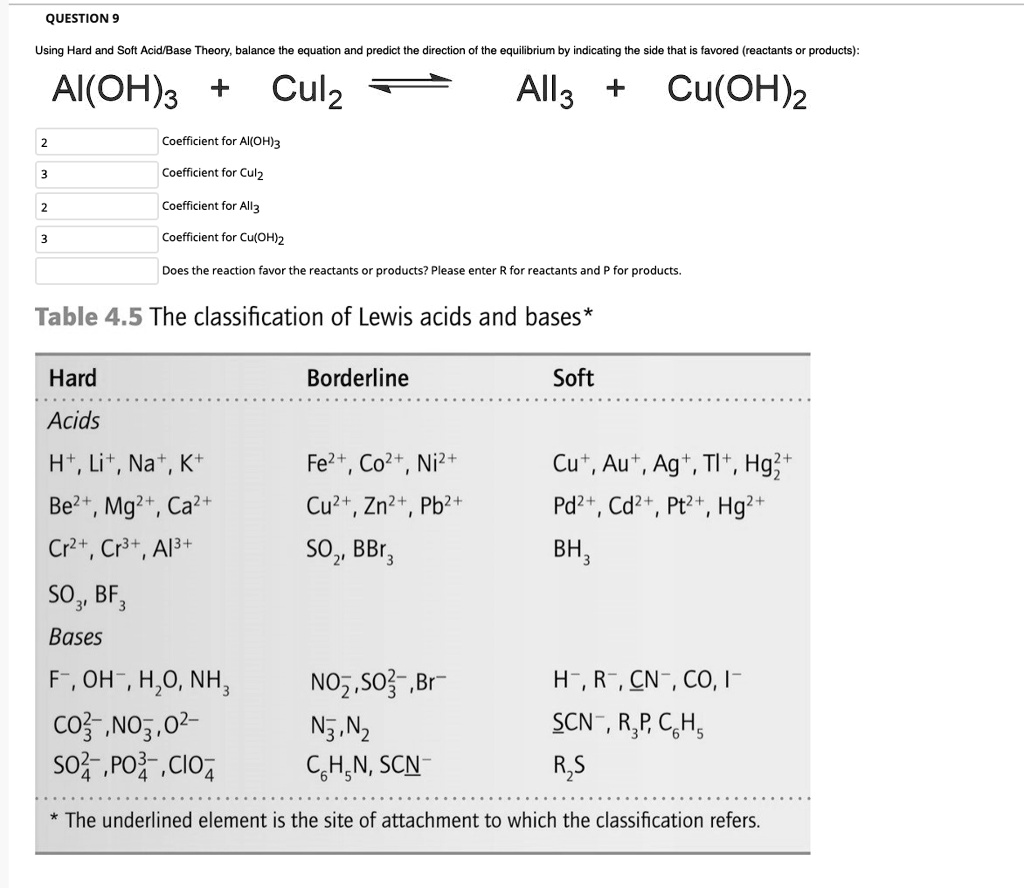
SOLVED: QUESTION Using Hard and Soft Acid Base Theory; balance the equation and predict the direction the equilibrium by indicating the side that is favored (reactants products): AI(OH)3 + Culz All3 +

Acids & Bases Acids: acids are sour tasting Arrhenius acid Arrhenius acid: Any substance that, when dissolved in water, increases the concentration. - ppt download
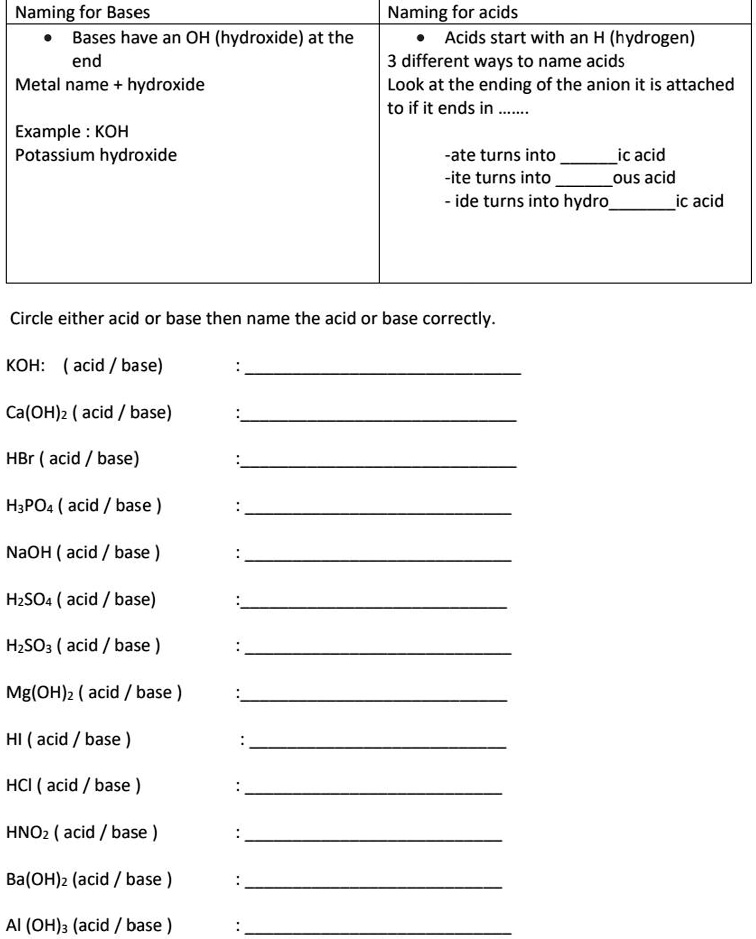
SOLVED: Naming for Bases Bases have an OH (hydroxide) at the end Metal name hydroxide Naming for acids Acids start with an H (hydrogen) 3 different ways to name acids Look at

Acids and Bases - the Three Definitions 1.Measurement of pH - the pH meter 2.Bronsted-Lowry definition of acids and bases - an acid is a proton donor - - ppt download
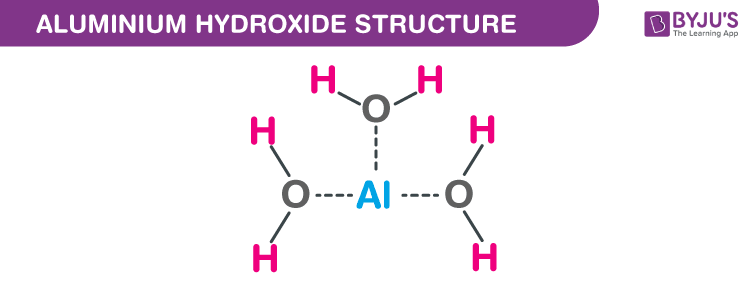
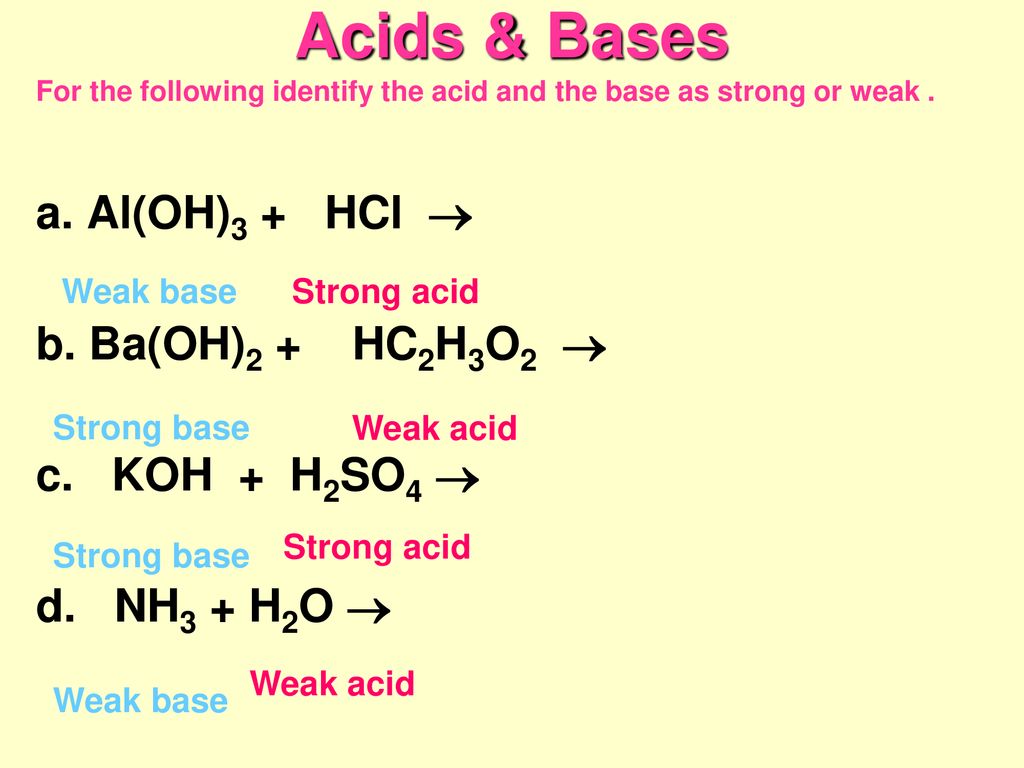
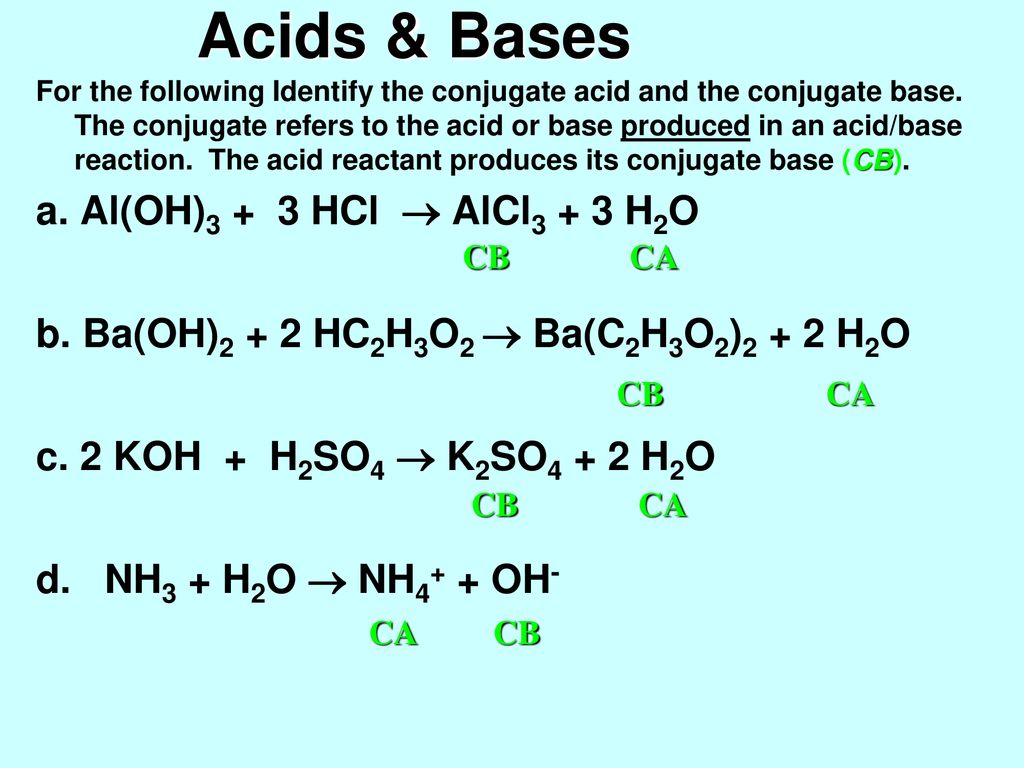
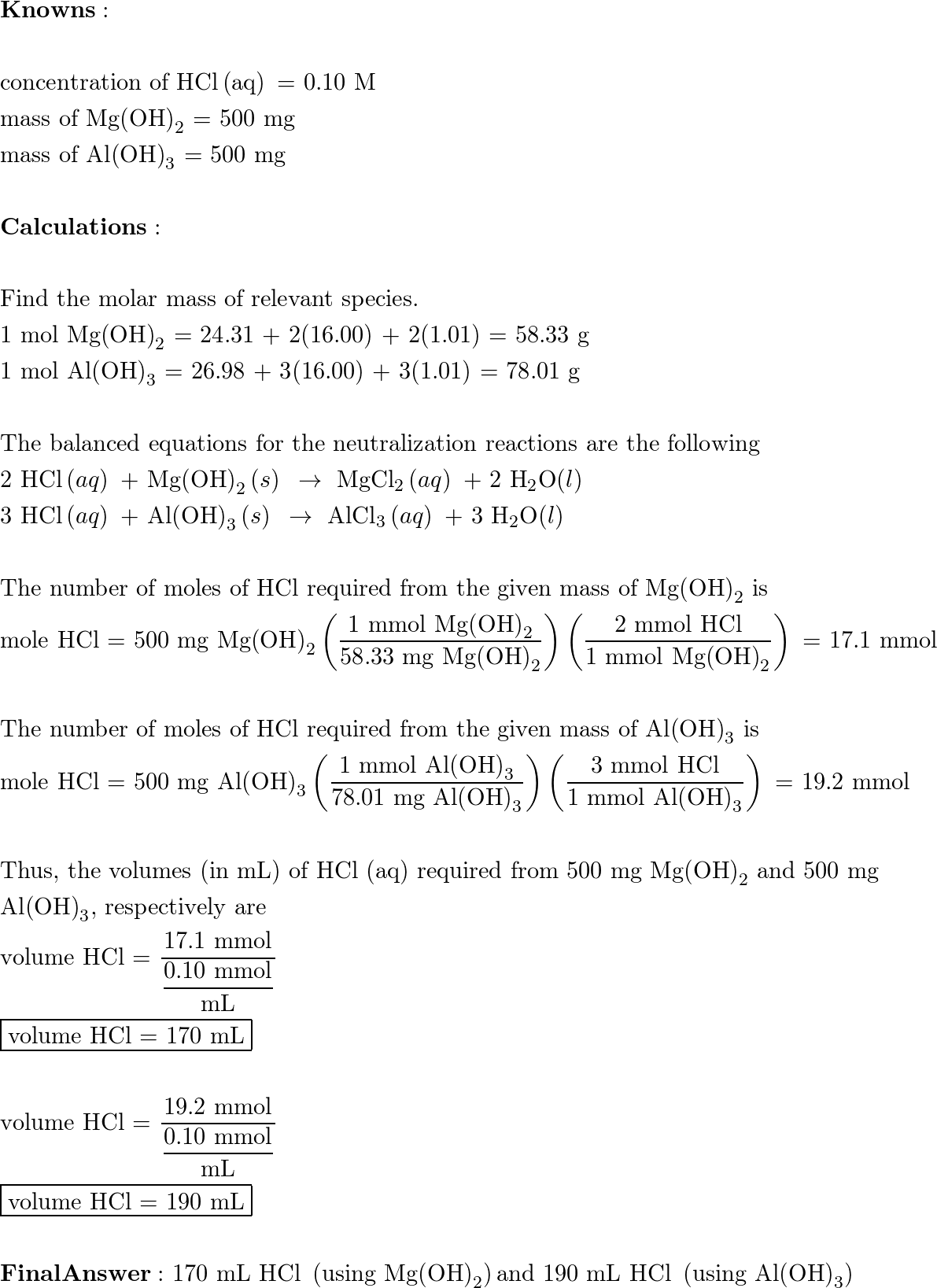
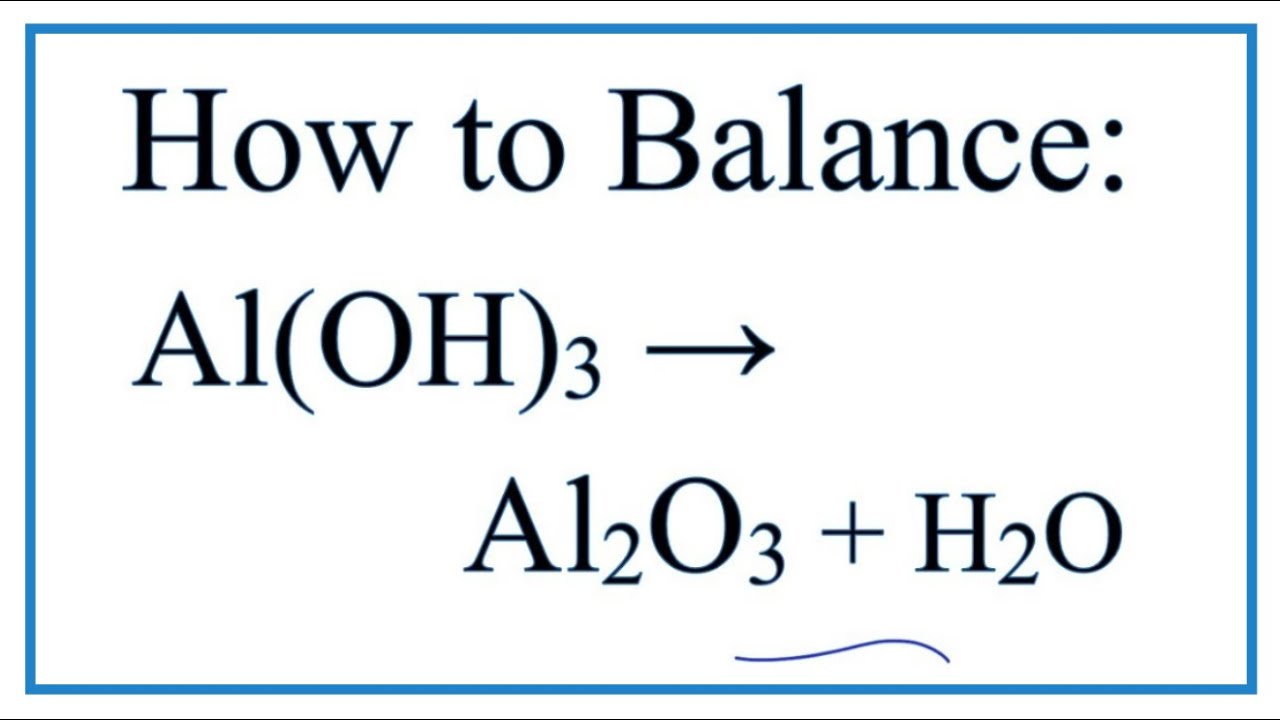
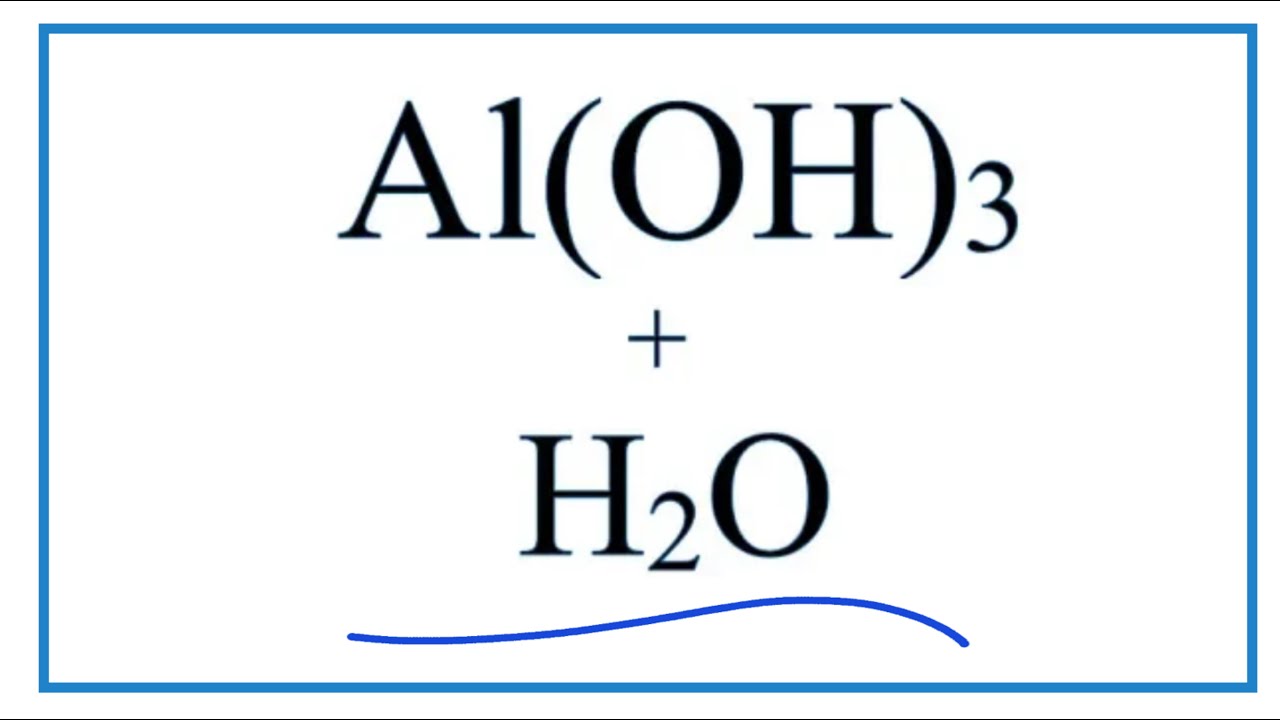
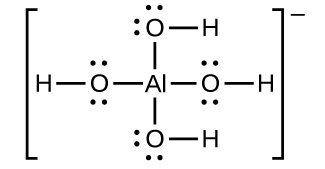

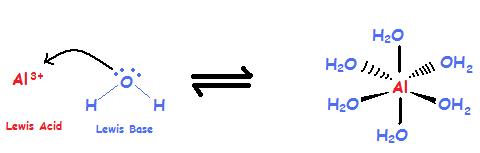
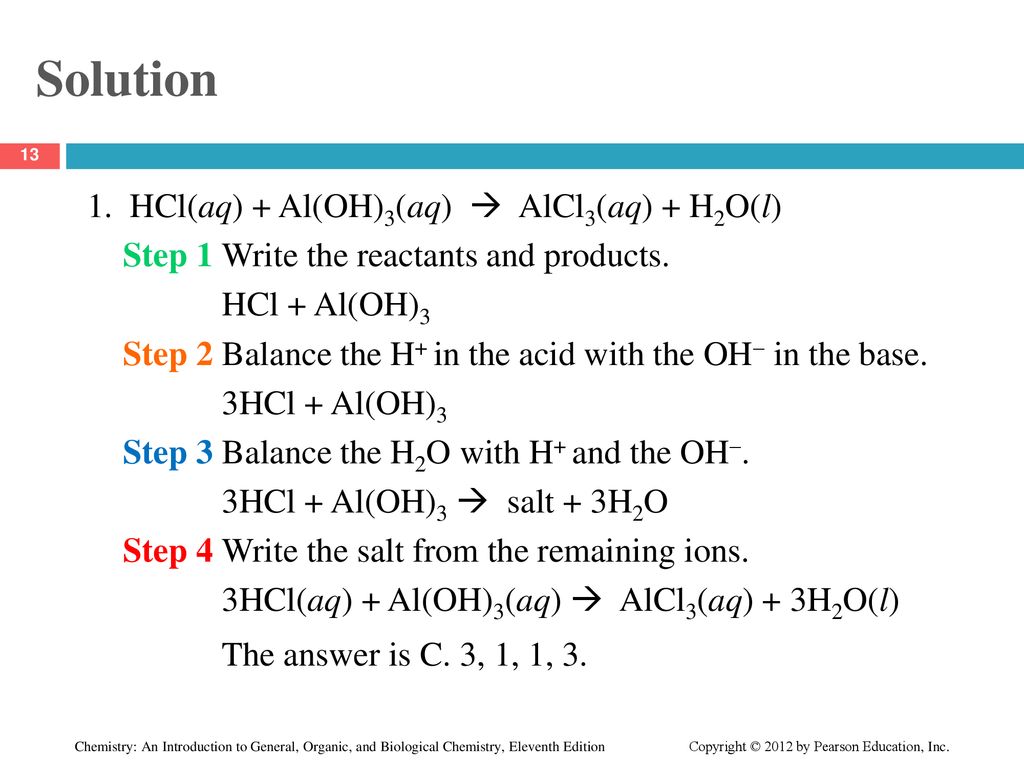


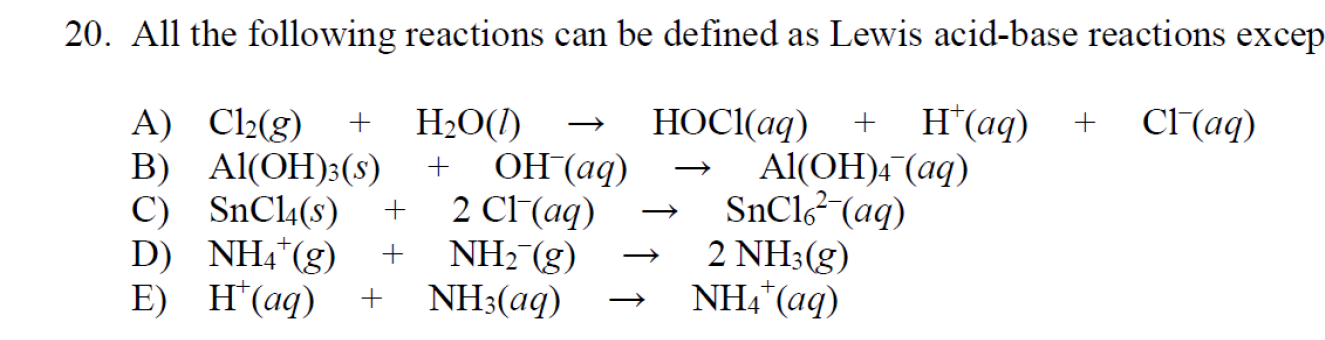
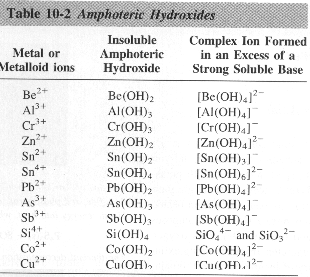

![Conjugate base of [Al(H(2)O)(6)]^(3+) is Conjugate base of [Al(H(2)O)(6)]^(3+) is](https://d10lpgp6xz60nq.cloudfront.net/ss/web/561545.jpg)