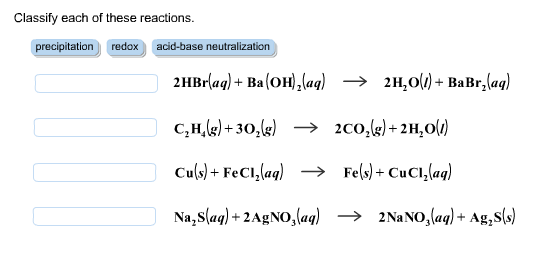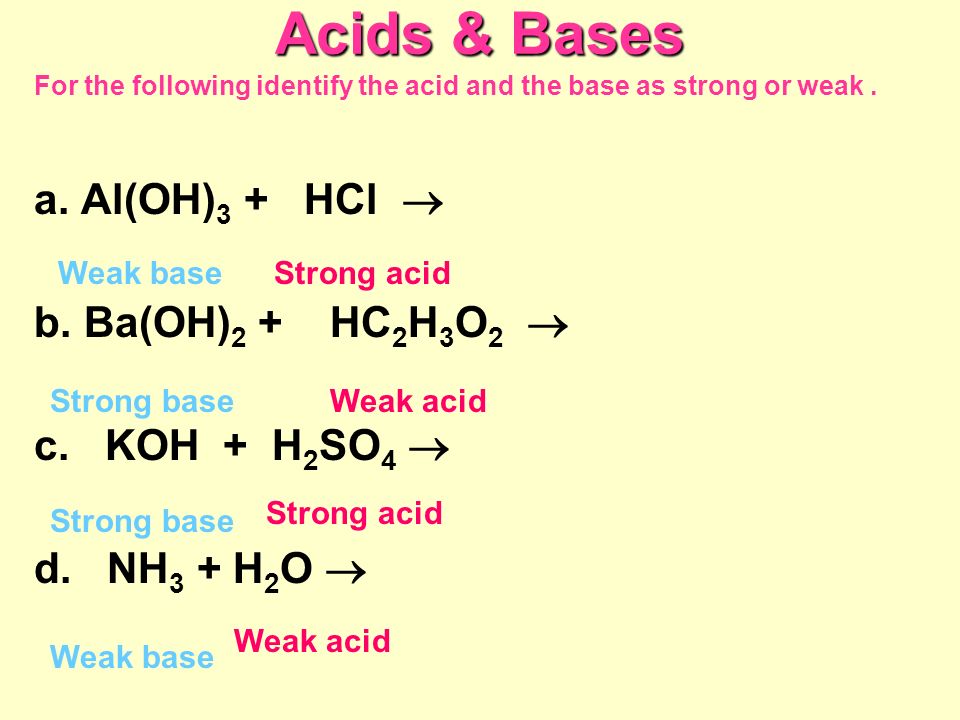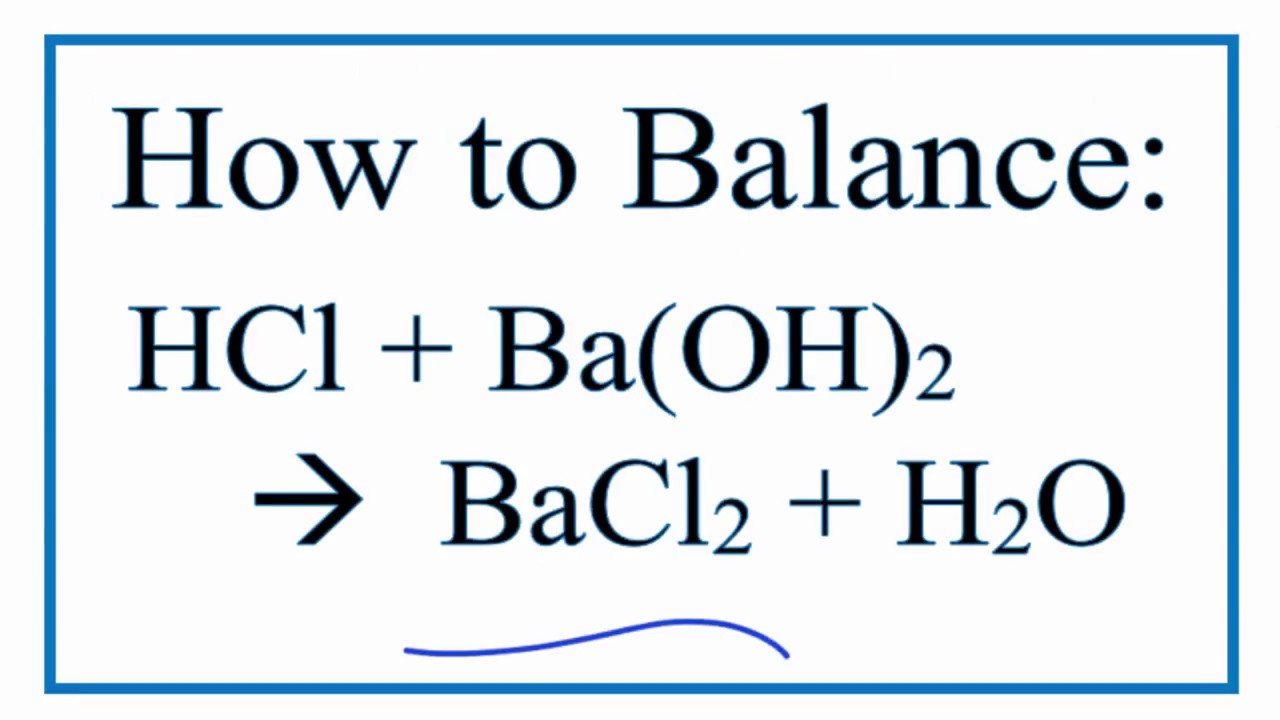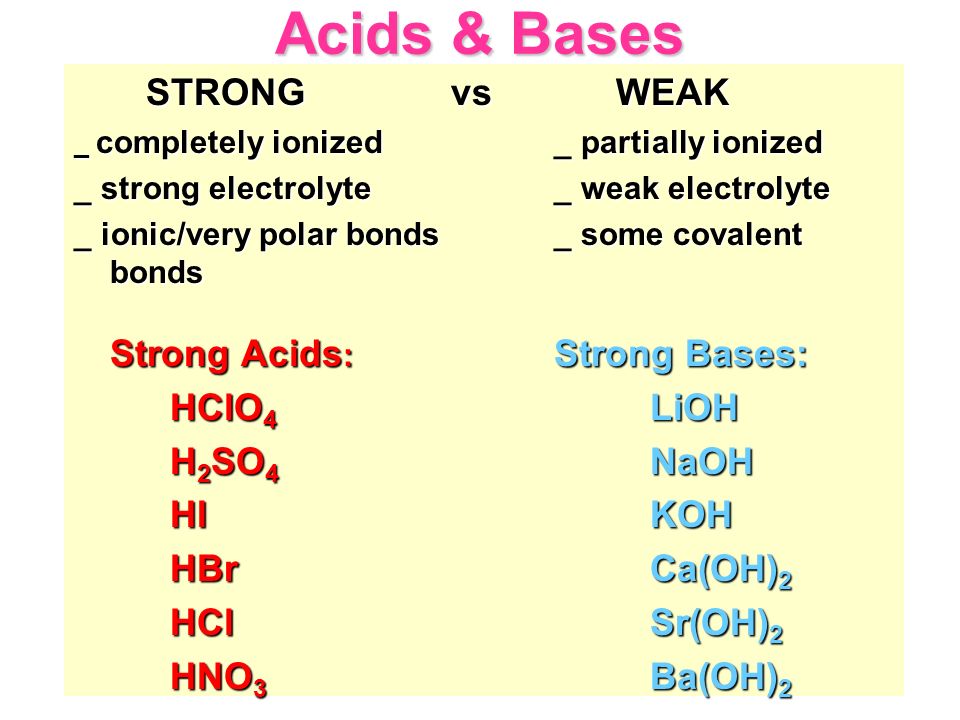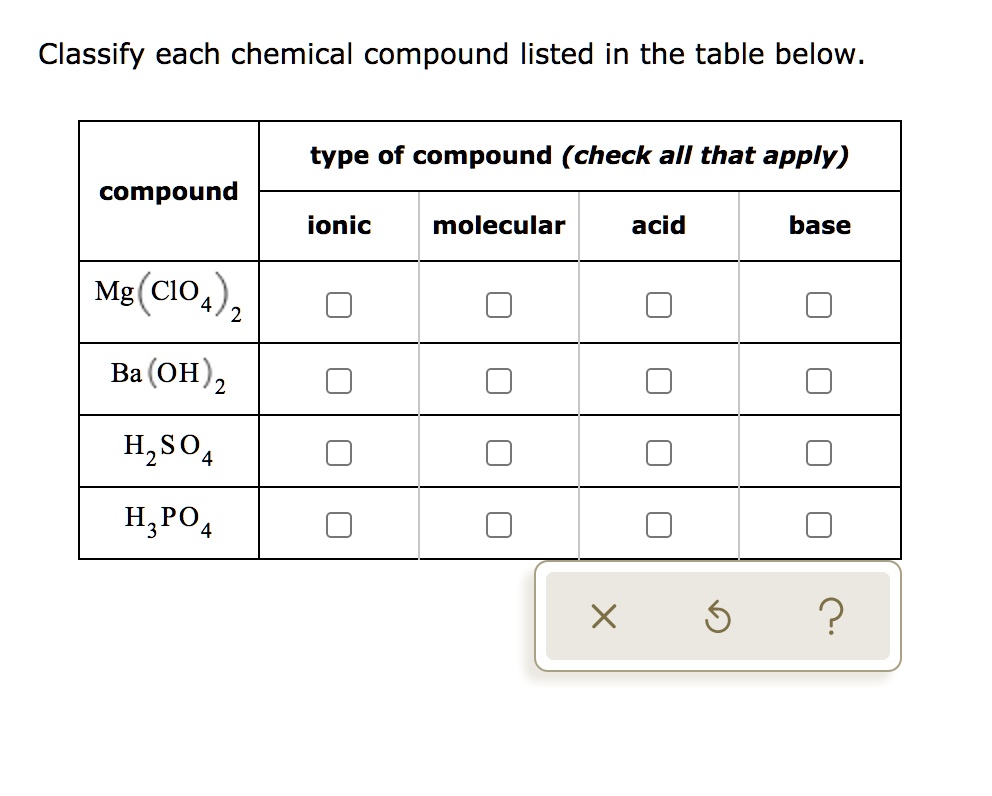
SOLVED: Classify each chemical compound listed in the table below type of compound (check all that apply) compound ionic molecular acid base Mg ClO Ba (OH) 2 H2S04 HzPO

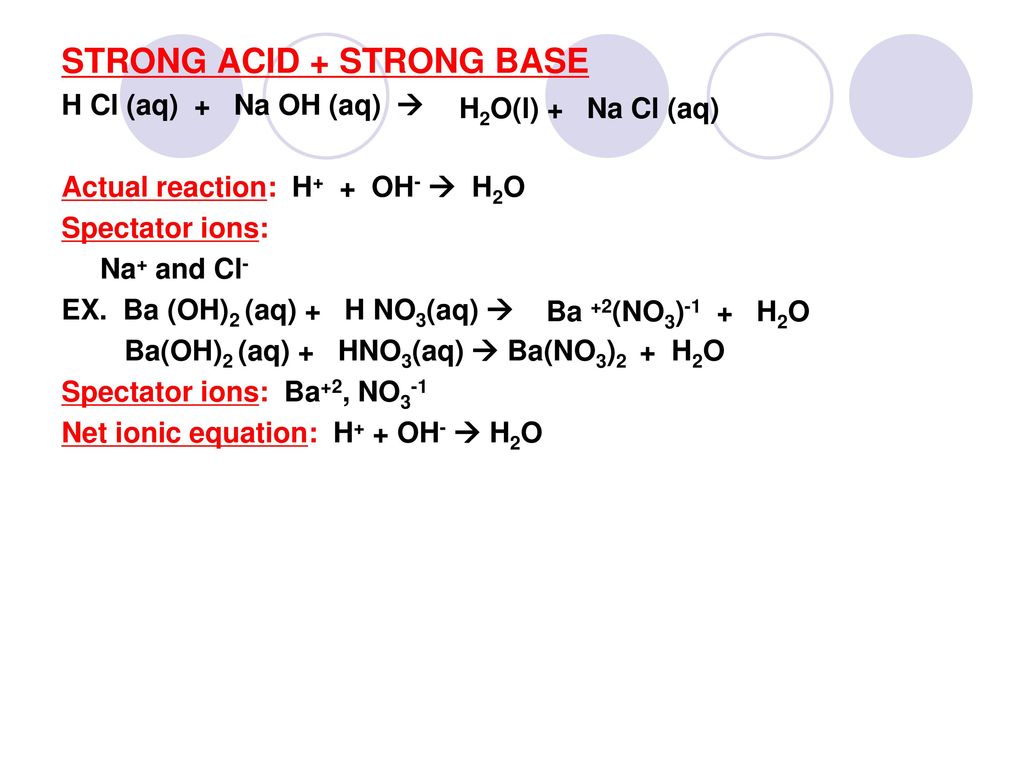
Acids & Bases Acids: acids are sour tasting Arrhenius acid Arrhenius acid: Any substance that, when dissolved in water, increases the concentration. - ppt download

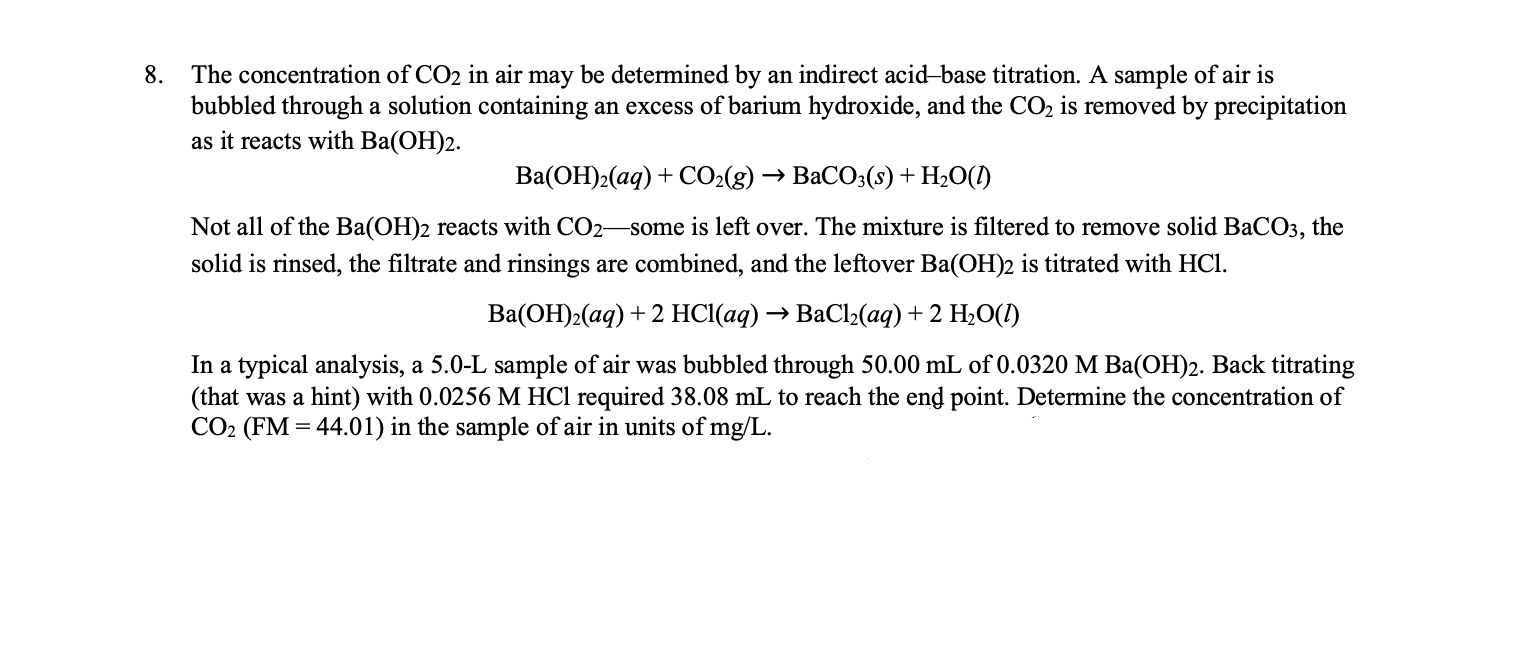
SOLVED: A solution of Ba(OH)2 was standardized against 0.1215 g of benzoic acid with grade of primary standard, C6H5COOH (122.12 g / mol). The end point was observed after adding 43.25 mL

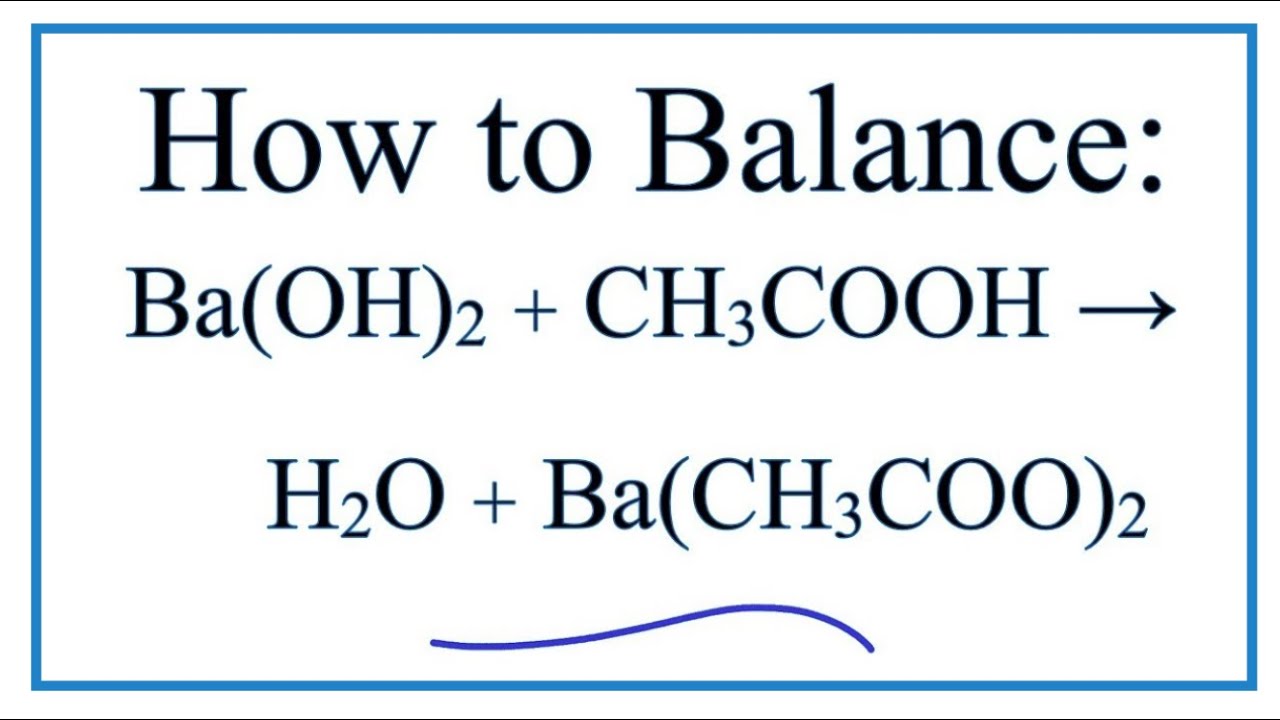
Question Video: Determining the Products of the Neutralization Reaction of Barium Hydroxide Ba(OH)₂ with Carbonic Acid H₂CO₃ | Nagwa