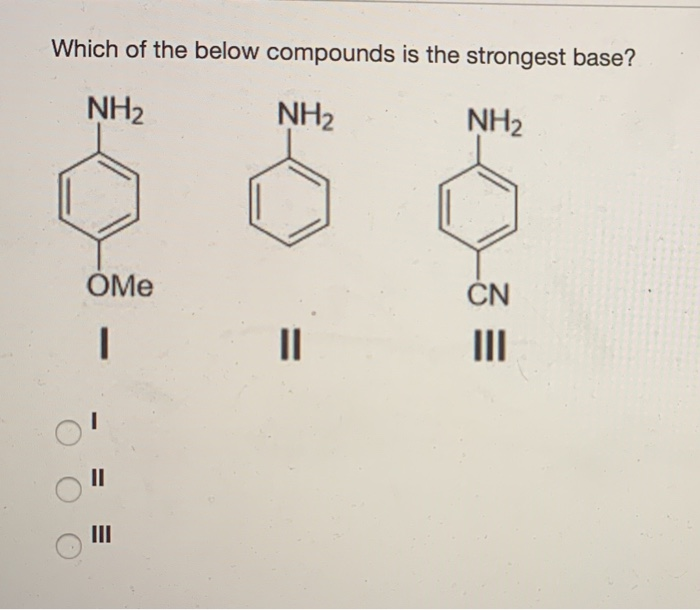
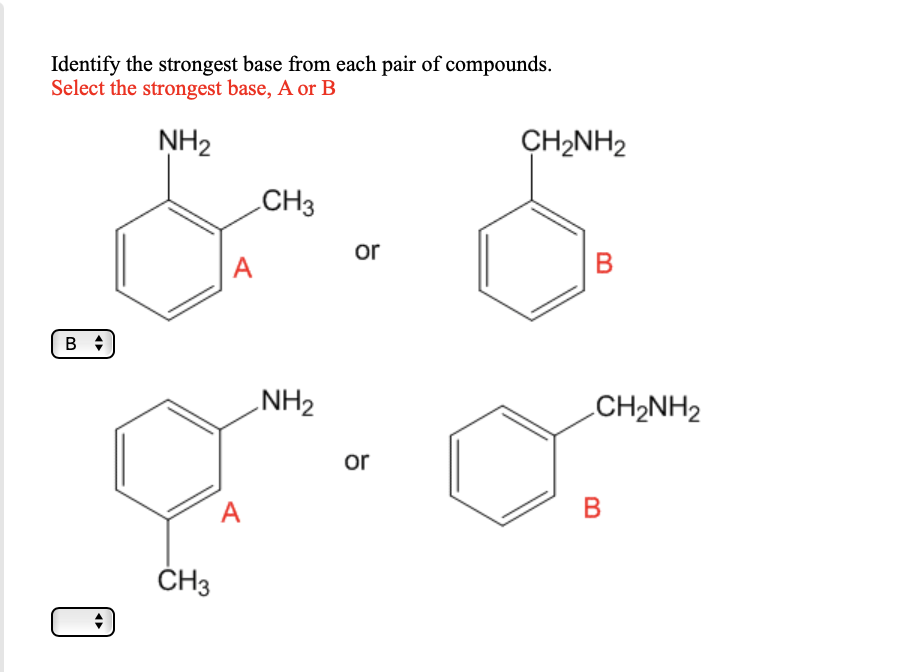
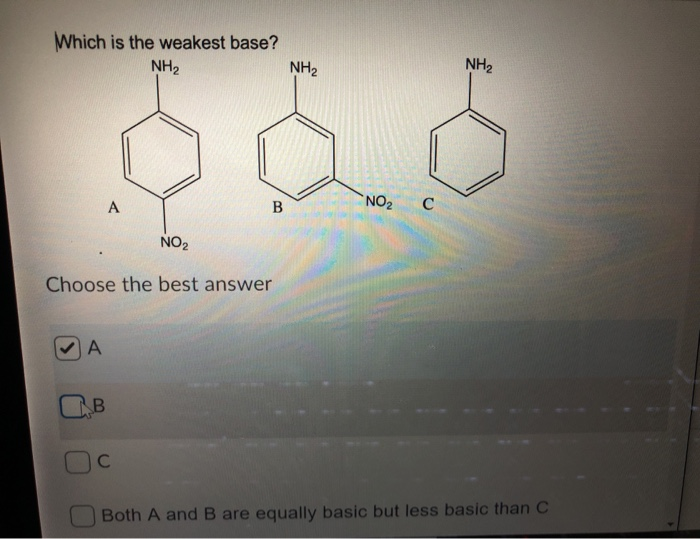
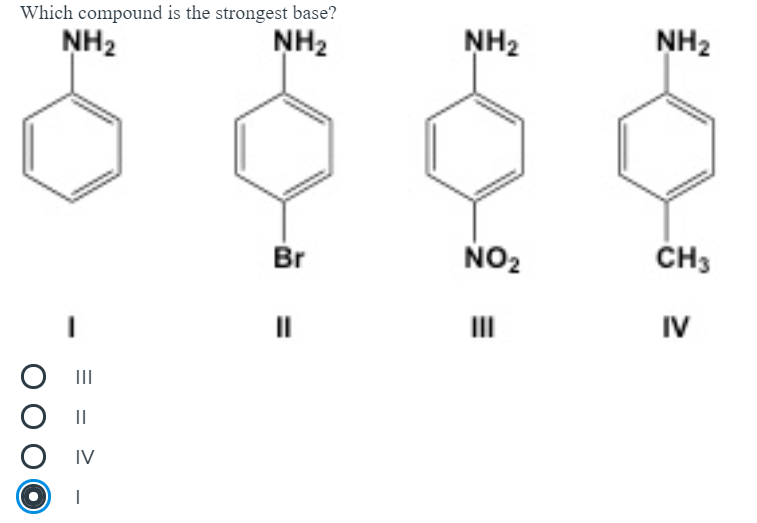
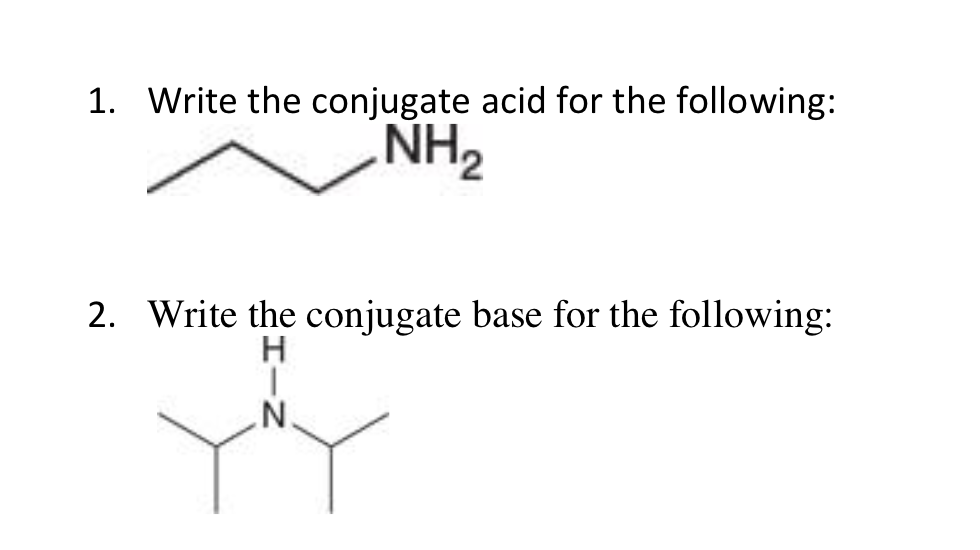
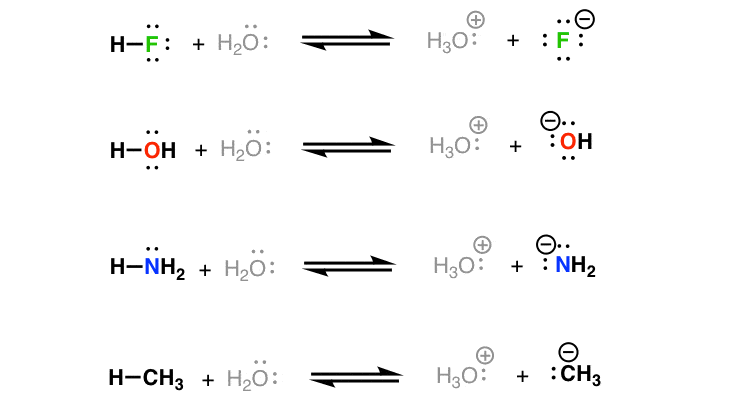
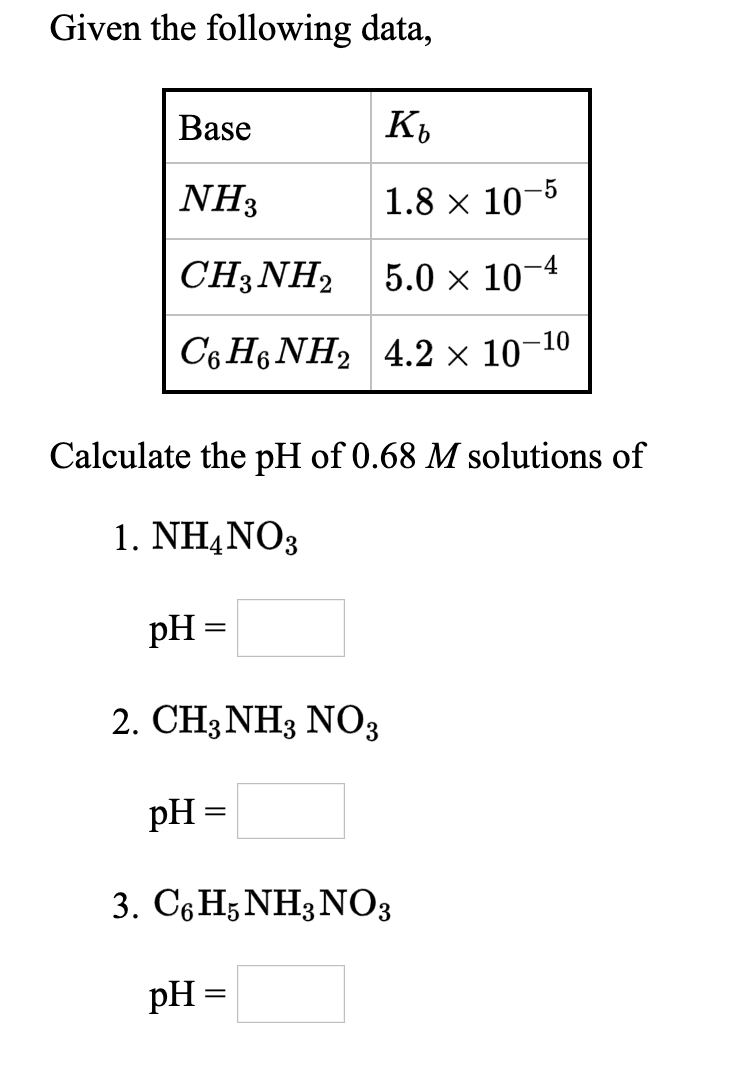
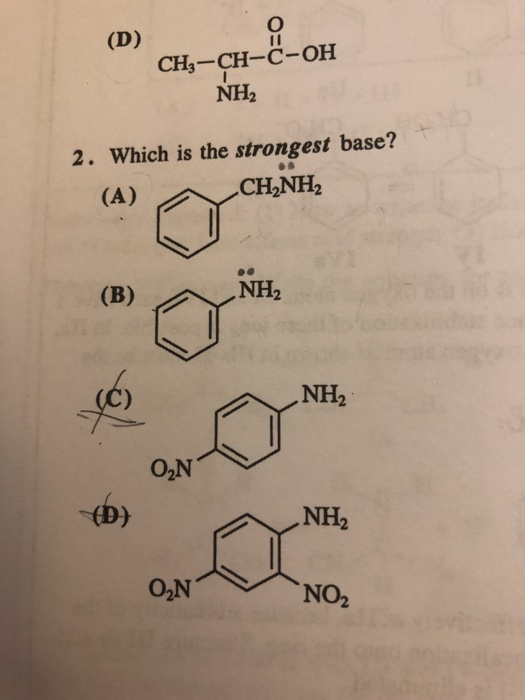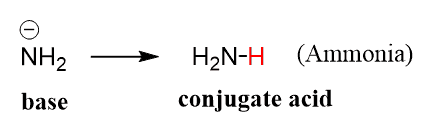OneClass: NH2 b. 1-(amino meth 㠗ょOH - 3. Select the stronger base from each pair of compounds Cl ...

China Top Quality Fuse Base and Fuse Link Nh00 Nh1 Nh2 Nh3 Nh4 Photos & Pictures - Made-in-china.com
![CH3 - OH|CH - CH2 - CH2 - NH2 [ Triethylamine ]Ethyl formate Product The major product of the given reaction is: CH3 - OH|CH - CH2 - CH2 - NH2 [ Triethylamine ]Ethyl formate Product The major product of the given reaction is:](https://dwes9vv9u0550.cloudfront.net/images/7494062/22483b28-e987-494e-85b6-898b531ad092.jpg)
CH3 - OH|CH - CH2 - CH2 - NH2 [ Triethylamine ]Ethyl formate Product The major product of the given reaction is:

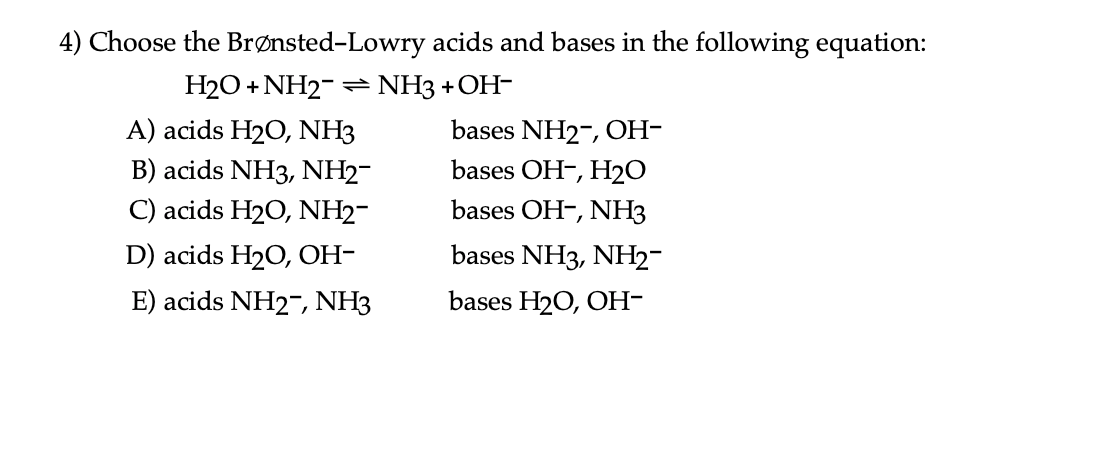
OneClass: Questions 3 and 4. Consider the reaction shown below: NH2-(ag) + H2O(l) ê·¼ NH3(gg) + OH-(a...

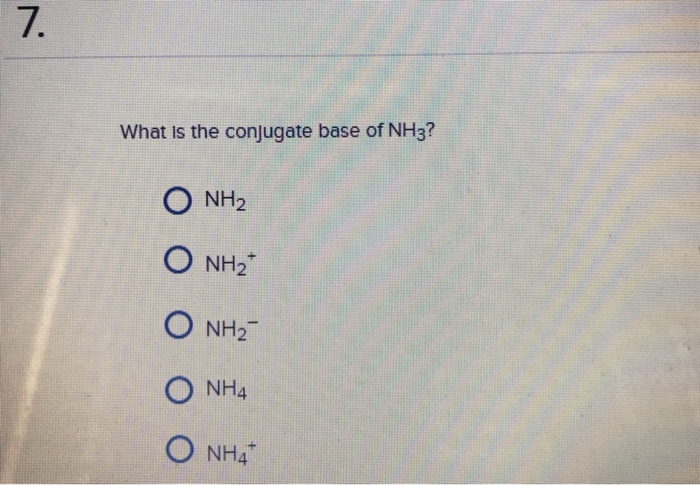
a) Mention conjugate base of each of the following: HS^-,H3O^+,H2PO4^-,HSO4^-,HF,CH3COOH,C6H5OH,HClO4,NH4^+ (b) Mention the conjugate acid of each of the following: OH^-,CH3COO^-,Cl^-,CO3^2 - ,H2PO4^-,CH3NH2,CH3COOH,NH2^- (c) Which of the following ...