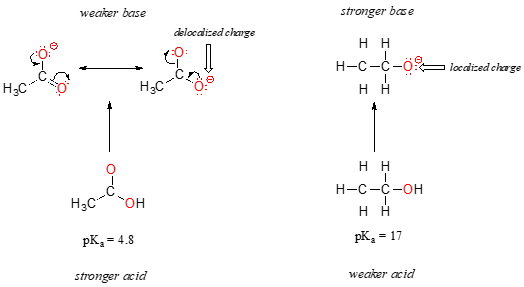
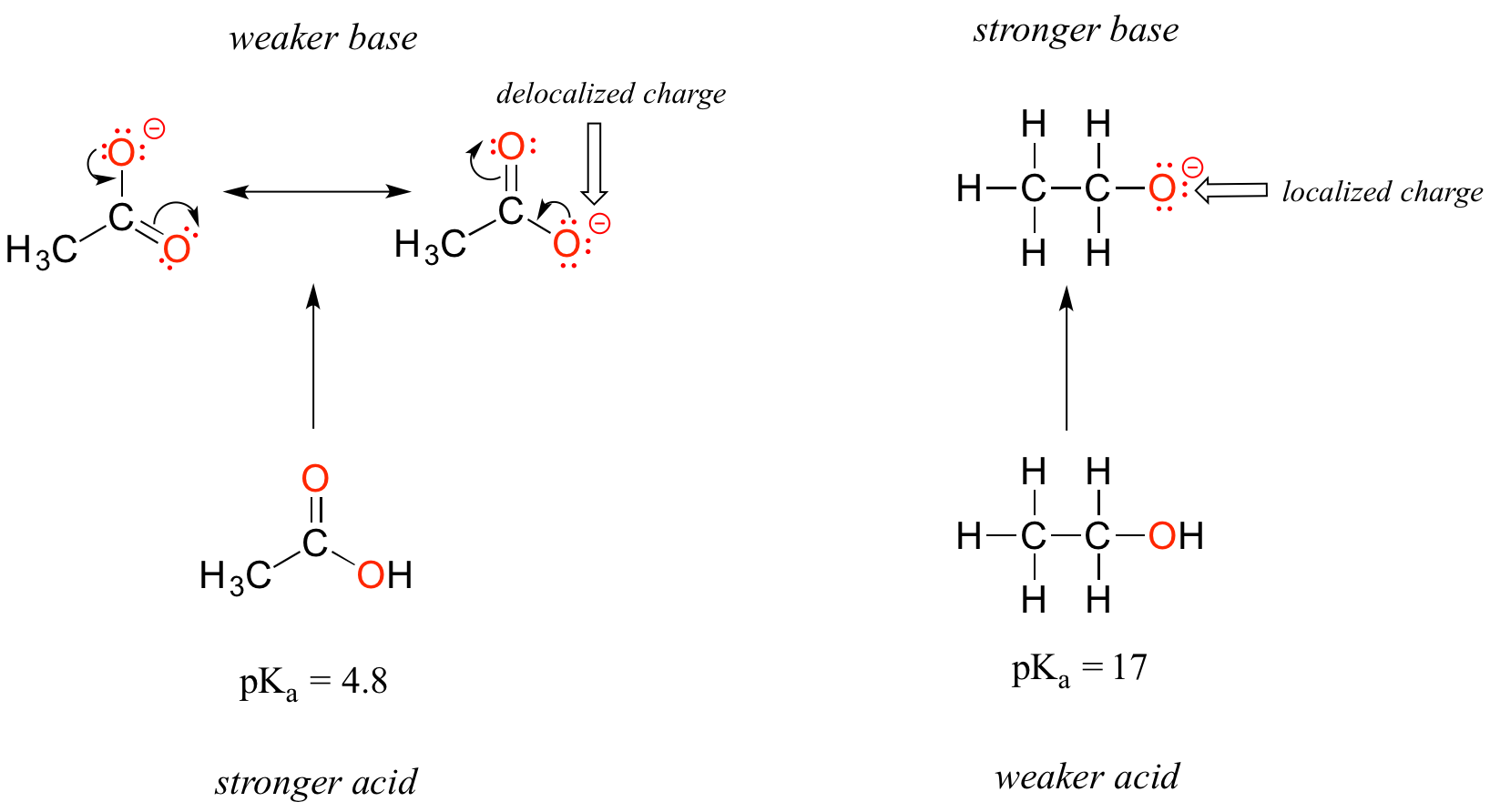
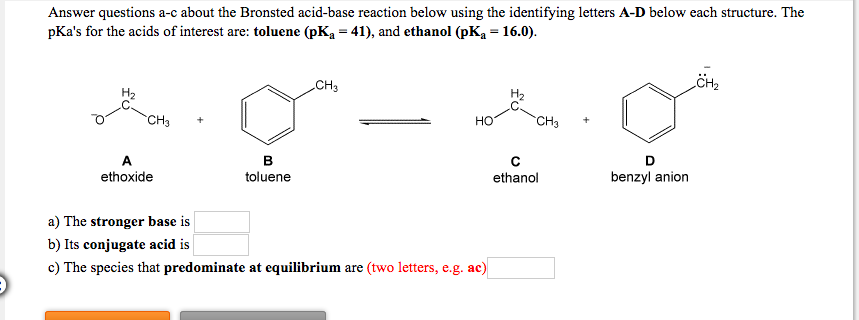
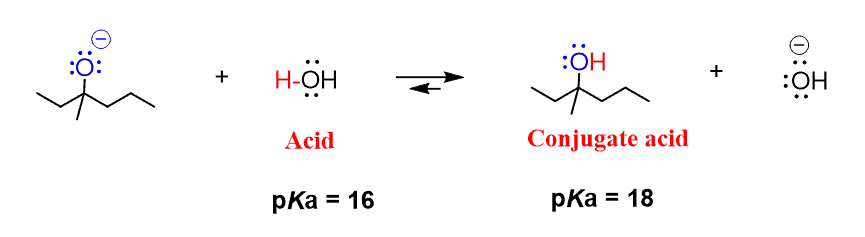
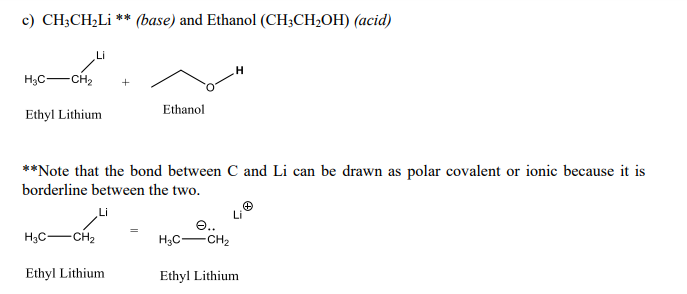
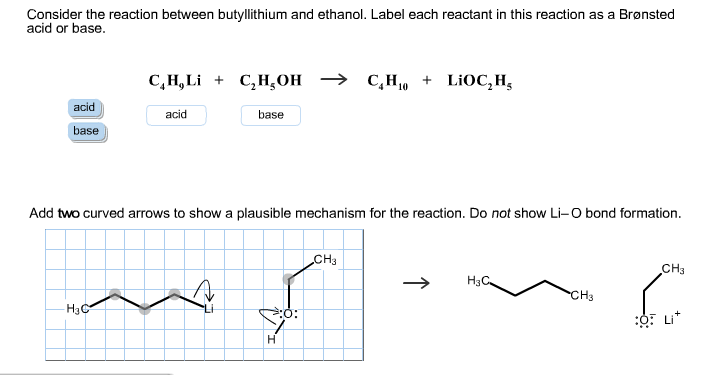
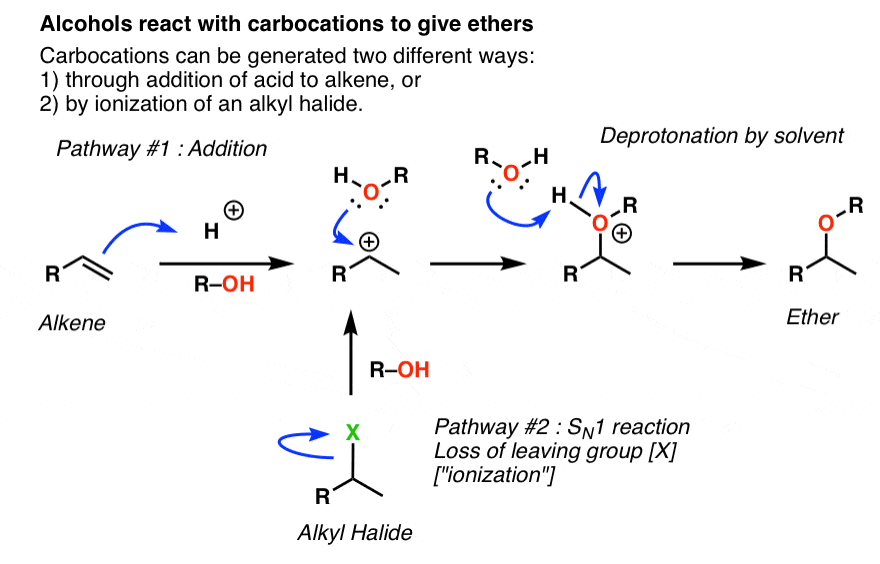
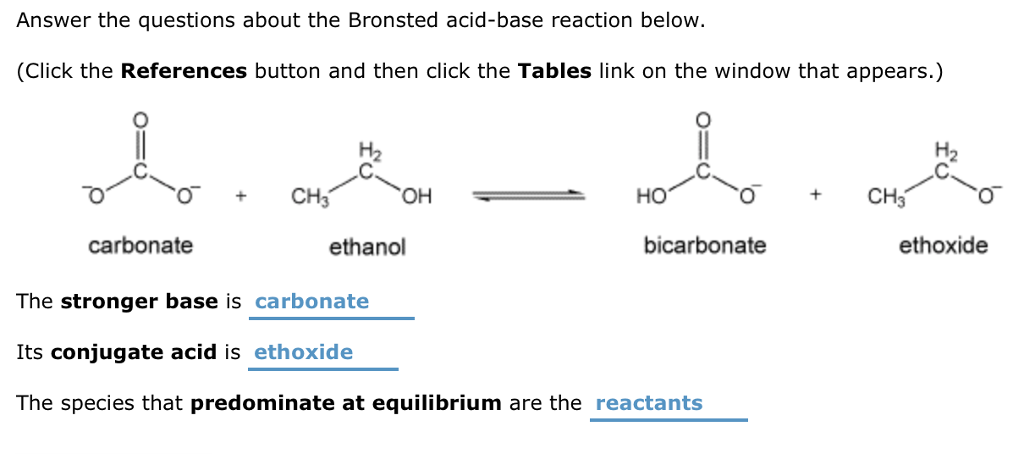
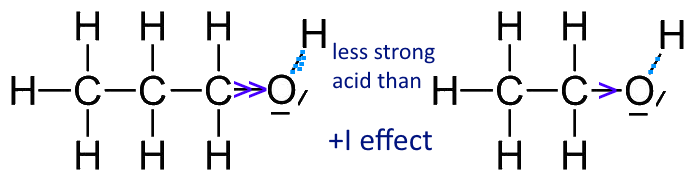
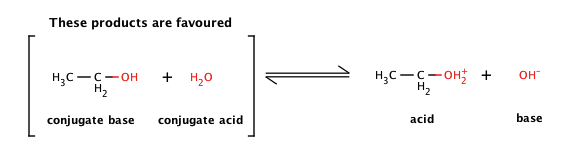
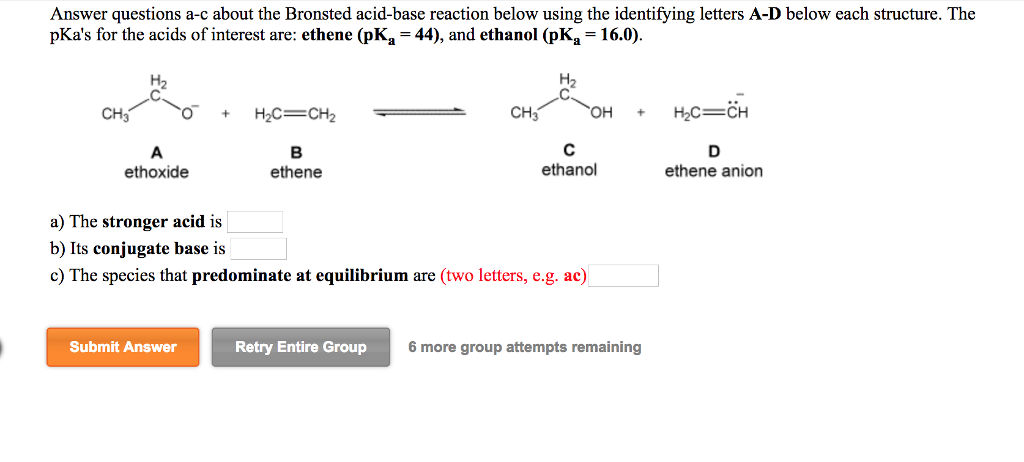
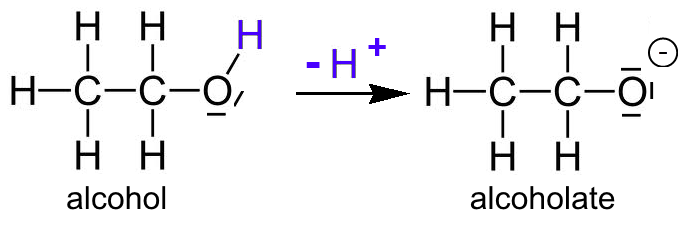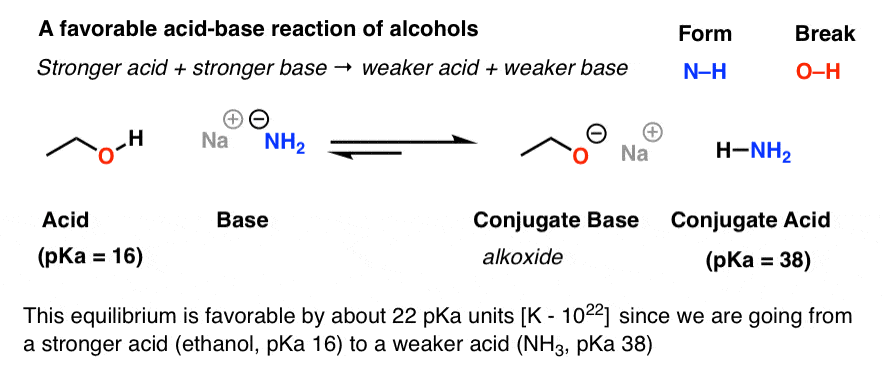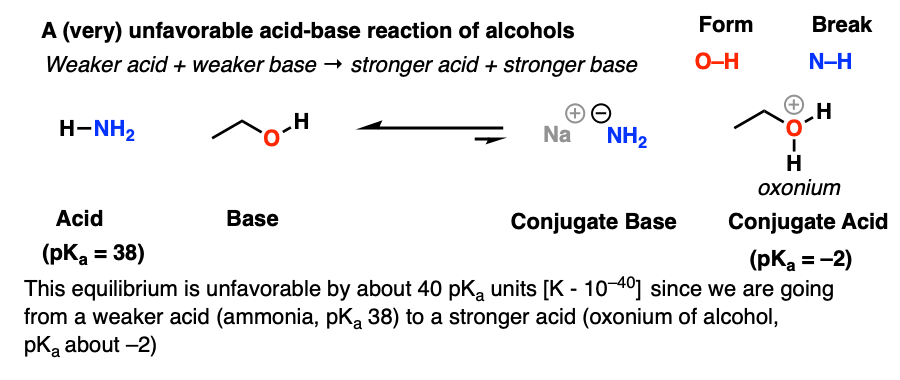Mechanism and Kinetics of Isobutene Formation from Ethanol and Acetone over ZnxZryOz | ACS Catalysis

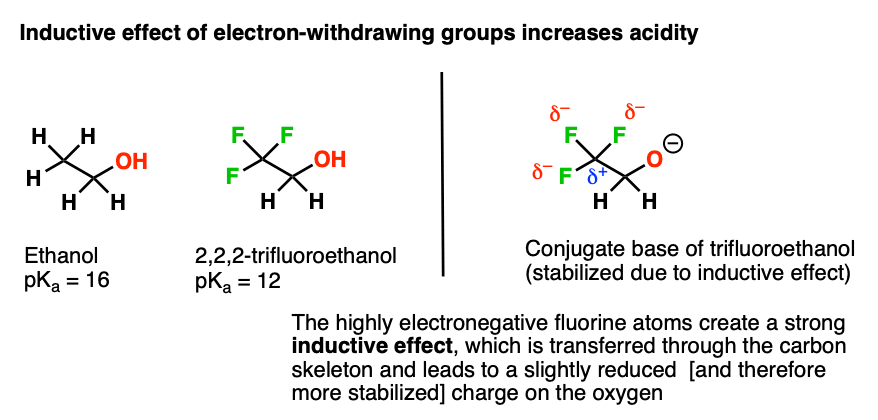
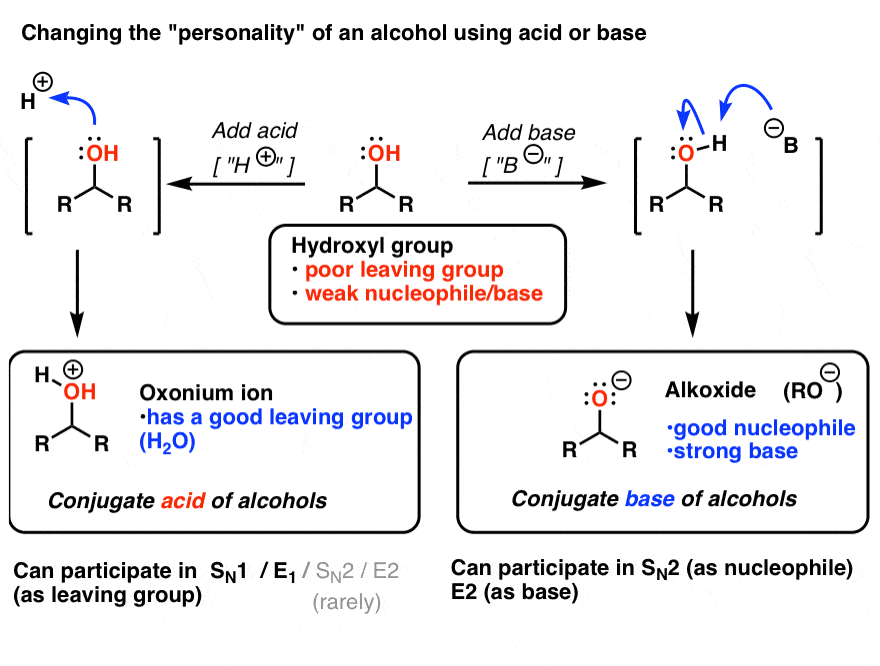
Why is the conjugate acid of an ether/alcohol more acidic than that of a hydronium ion? : r/chemhelp

Key Roles of Lewis Acid–Base Pairs on ZnxZryOz in Direct Ethanol/Acetone to Isobutene Conversion | Journal of the American Chemical Society