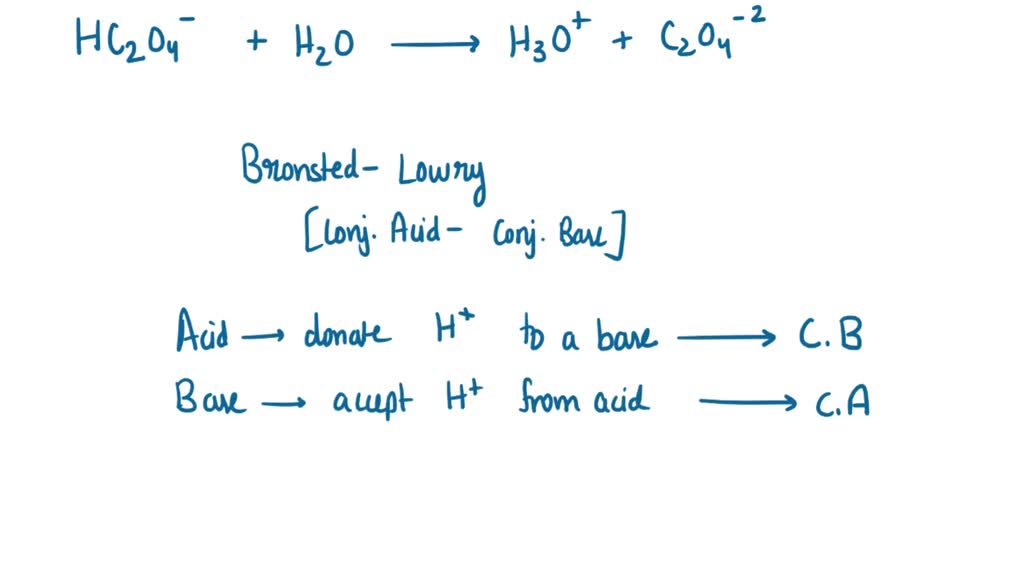Bouteille de sport H2O Active® Base Tritan™ de 650 ml à couvercle à clapet | Gourde publicitaire | Printecom.fr

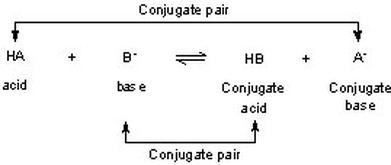
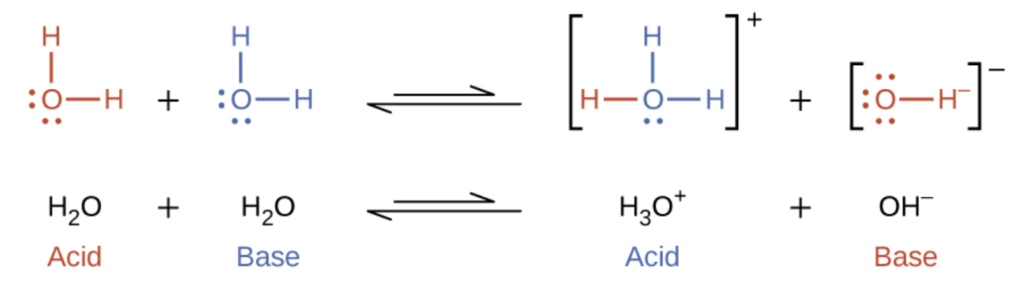
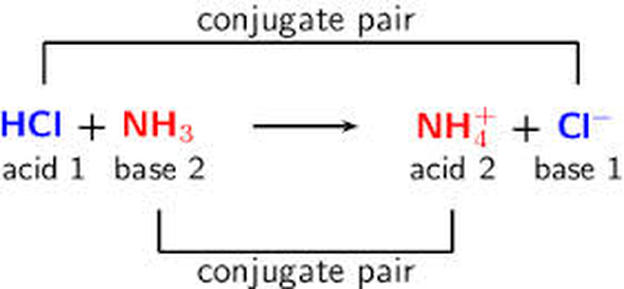
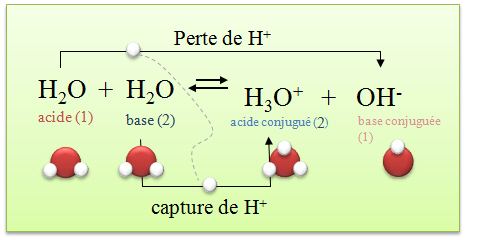
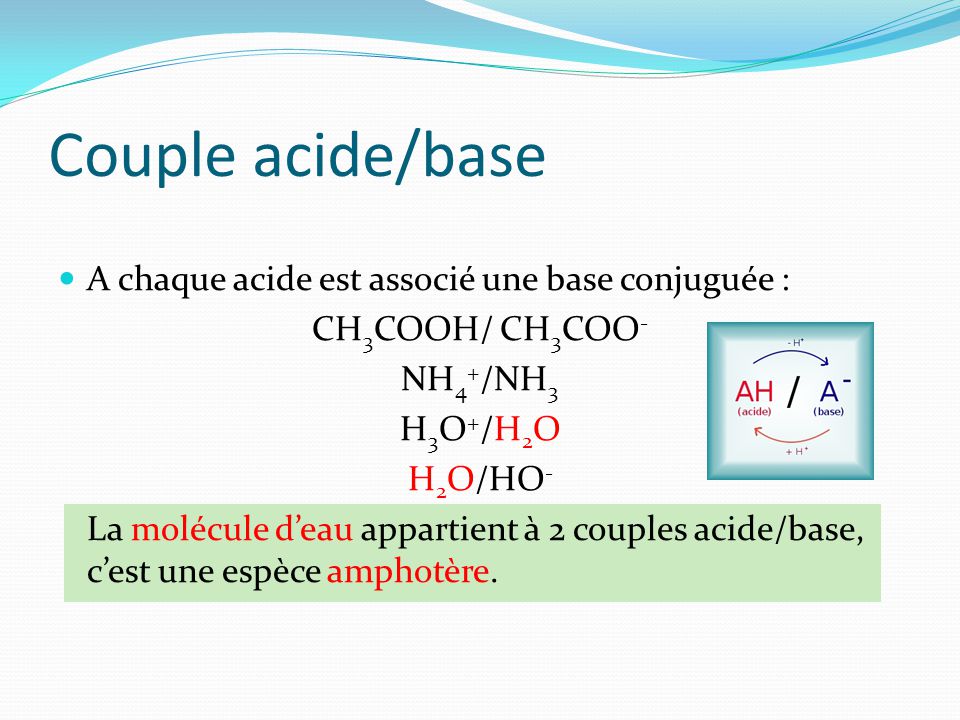
G) Sels Dans le chapitre 1, Acide + Base Sel + Eau HCl(aq) + NaOH(aq) NaCl(aq) + H2O Les sels sont des électrolytes forts qui se dissocient entièrement. - ppt video online télécharger
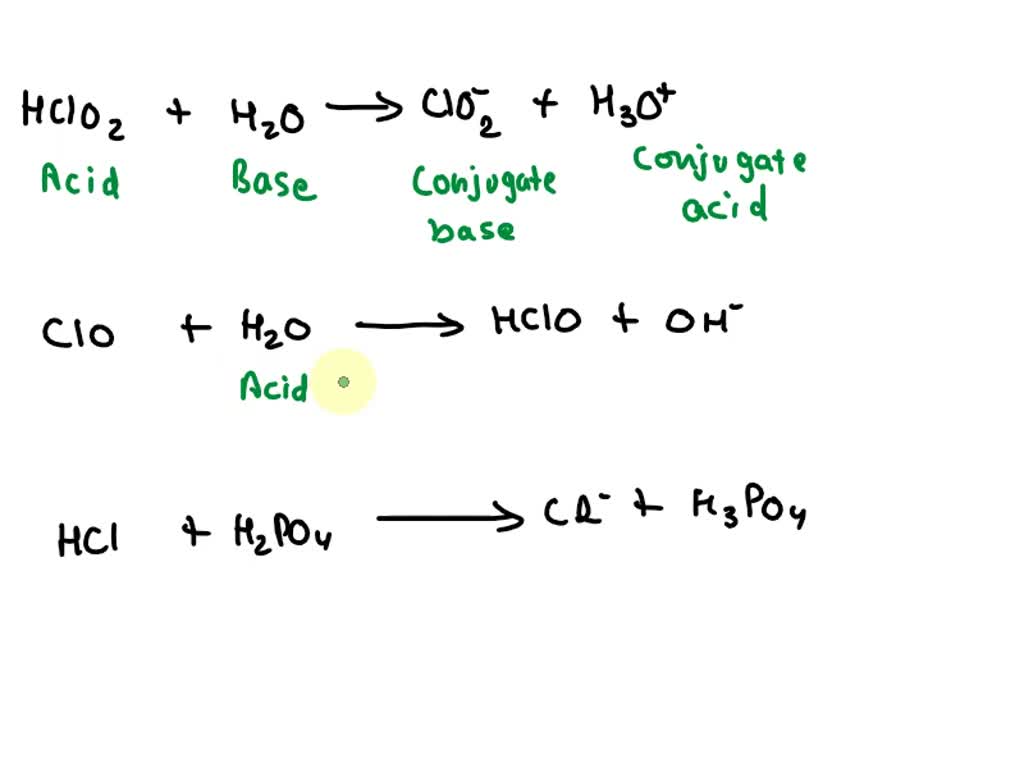
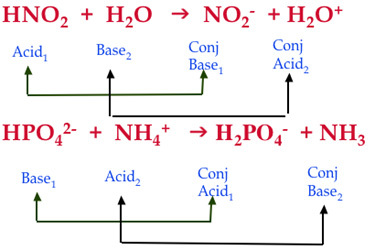
SOLVED: Complete the table Equation Acid Base Conjugate base Conjugate acid HClO2 + H2O → ClO−2 + H3O+ ClO− + H2O → HClO + OH− HCl + H2PO−4 → Cl− + H3PO4

Réaction Acide-base. Réaction Chimique Neutralisant Les Propriétés Acides Et Basiques, Produisant Un Sel Et De L'eau. Utilisé Pour Déterminer Le Ph. Bronsted - Théorie De Lowry. Molécules De Hcl, Naoh, H2o Et