
Which of the species from the equilibrium below are conjugate acid-base pairs? NH3(aq) + H2S(aq) arrow HS-(aq) + NH4+(aq) | Homework.Study.com
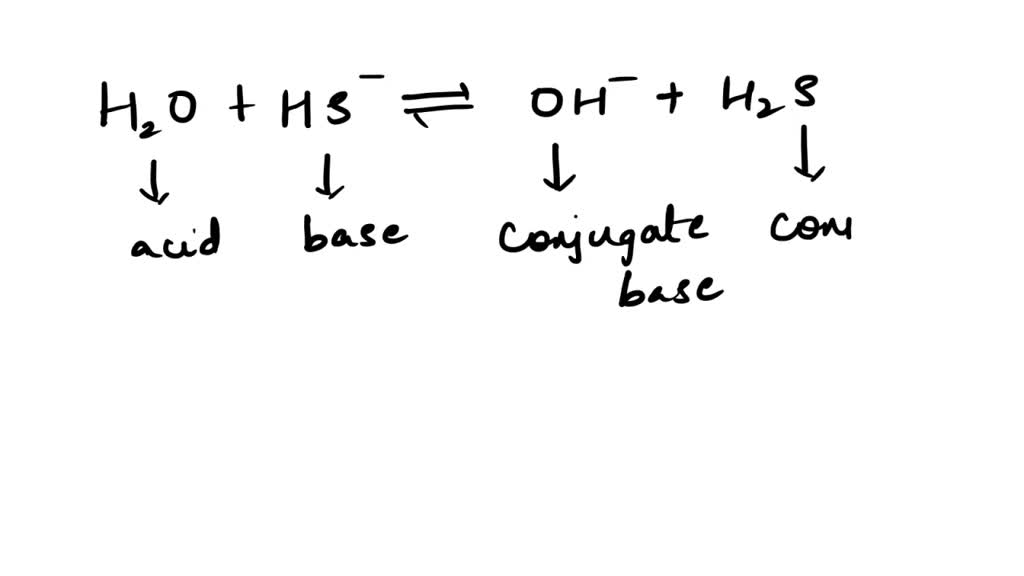
SOLVED: Be sure to answer all parts. In the following equation, identify the acids, bases, and conjugate pairs. H2O + HS− ⇌ OH− + H2S (a) What is the acid? H2O HS−
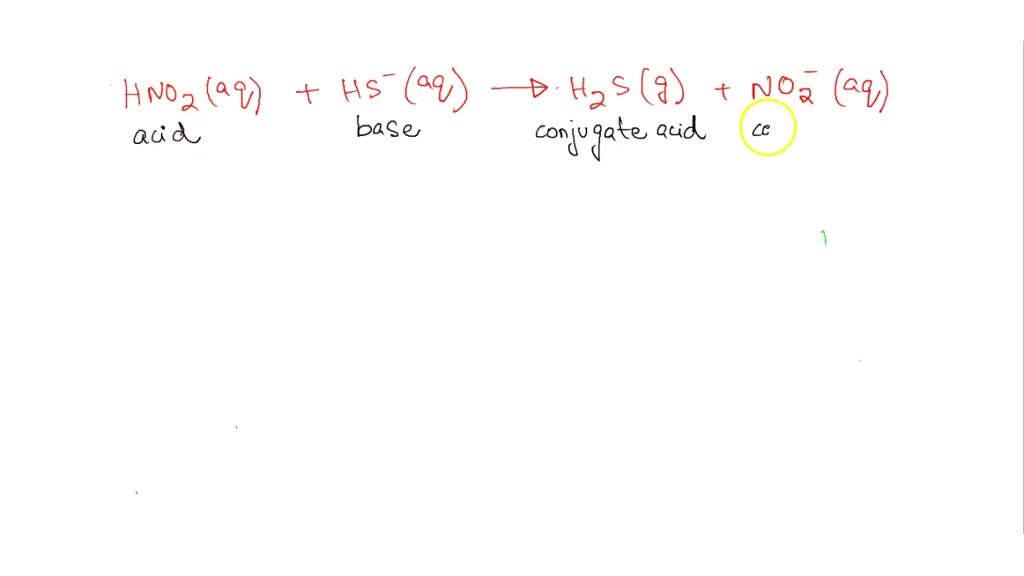
SOLVED: Consider the following reaction. Which are conjugate acid/base pairs? HNO2(aq) + HS-(aq) —-> H2S(g) +NO2-(aq) Group of answer choices H2S NO2^- HNO2 HS^- HS^- NO2^- HNO2 NO2^- HNO2 H2S

Hydrogen Sulfide: Chemical Biology Basics, Detection Methods, Therapeutic Applications, and Case Studies | Wiley



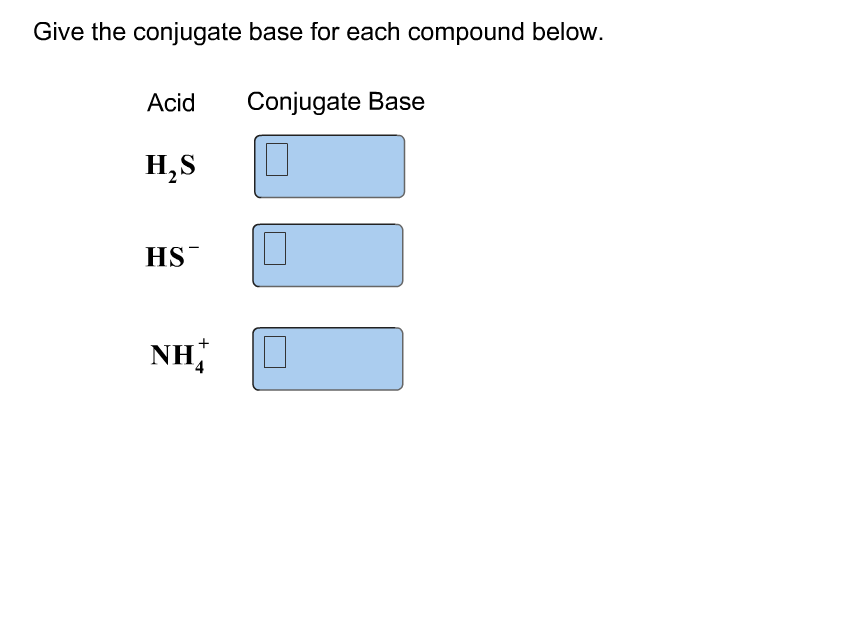



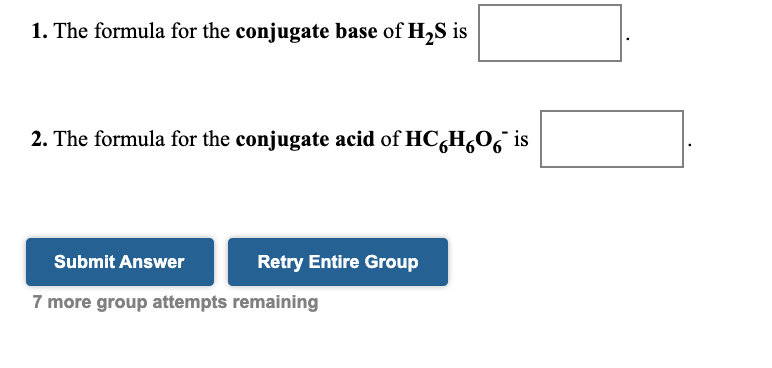
.jpg)










