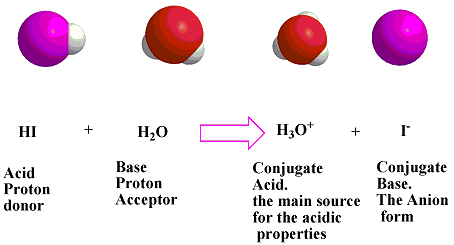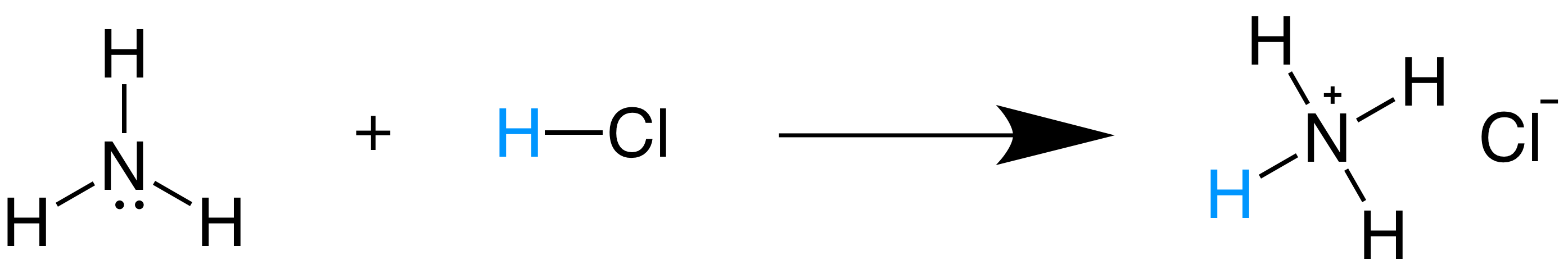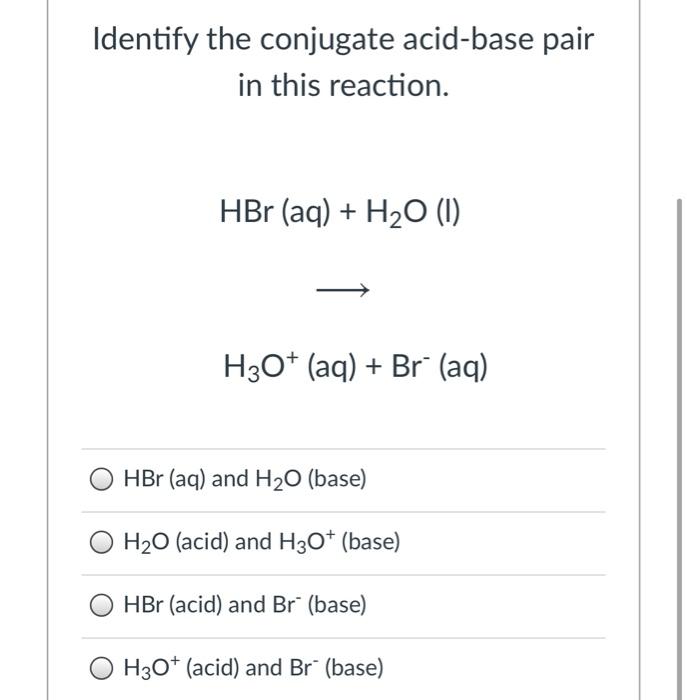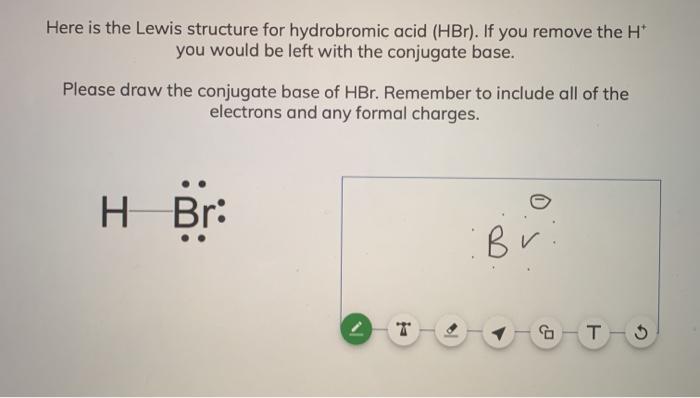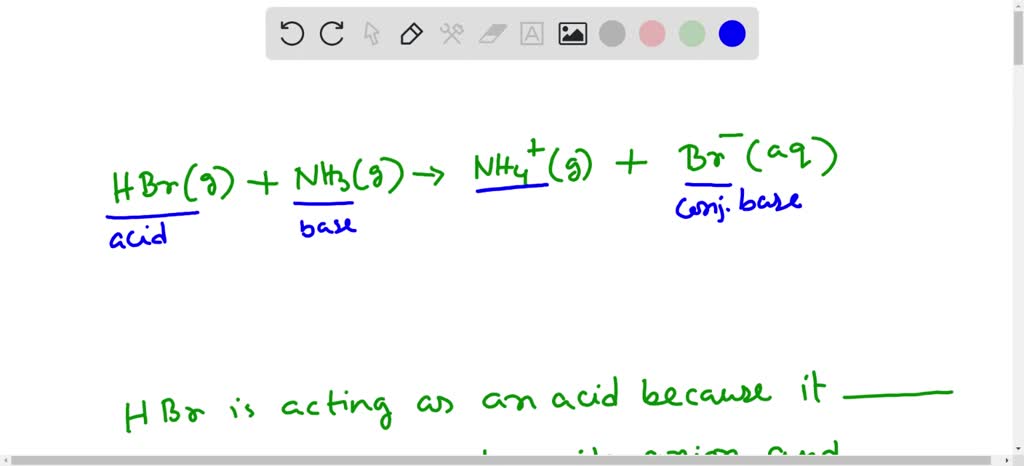
SOLVED: In the following acid-base reaction: HBr(g) + NH3(g) → NH4+(aq) + Br– (aq) HBr is acting as the acid, because it a proton to form bromide anion, and NH3 is acting
1 Introduction to Acids and Bases The earliest definition was given by Arrhenius: An acid contains a hydrogen atom and dissolves in water to form a hydrogen. - ppt download
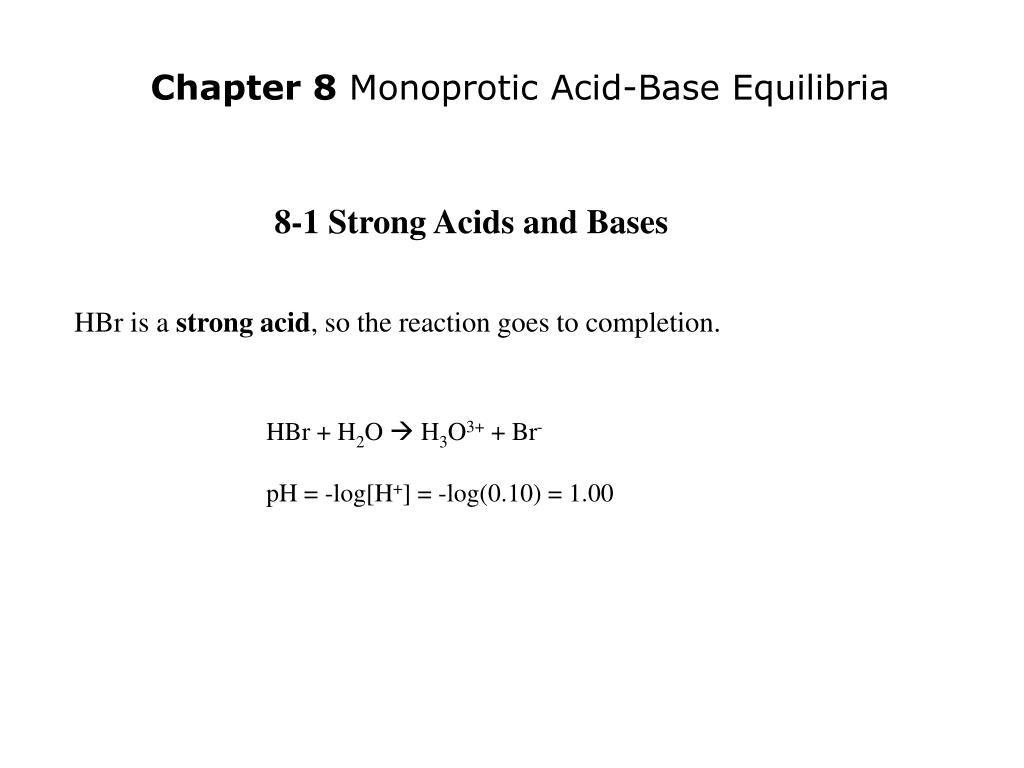
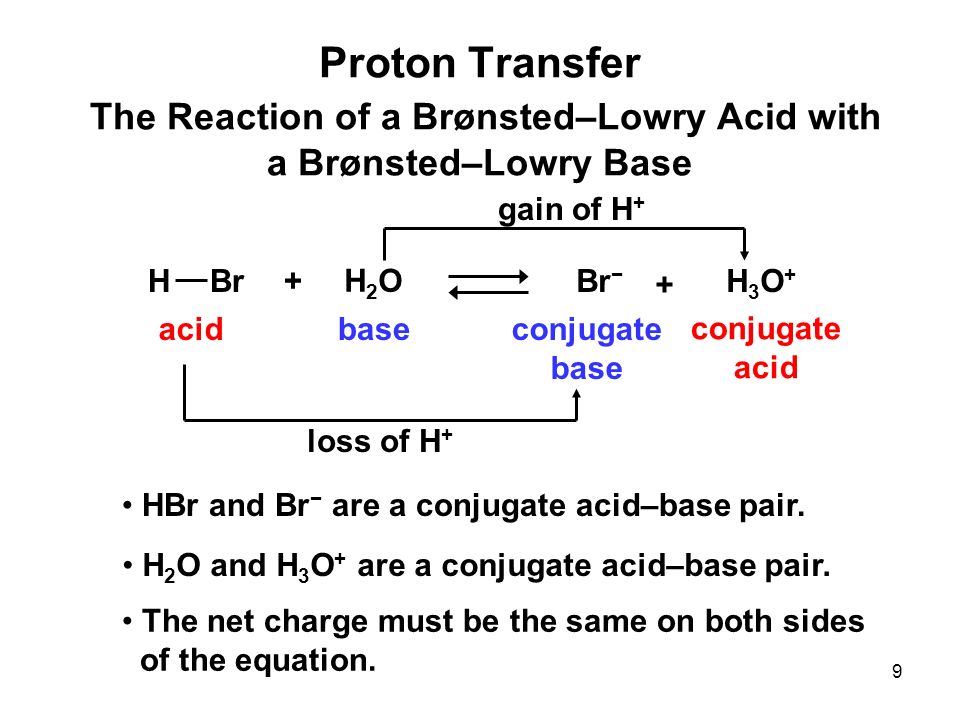
PPT - HBr is a strong acid , so the reaction goes to completion. PowerPoint Presentation - ID:4536389
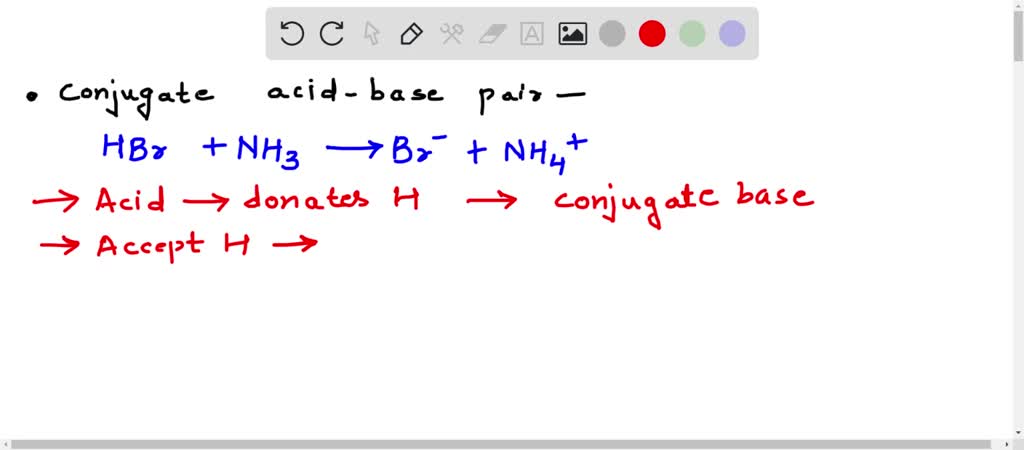
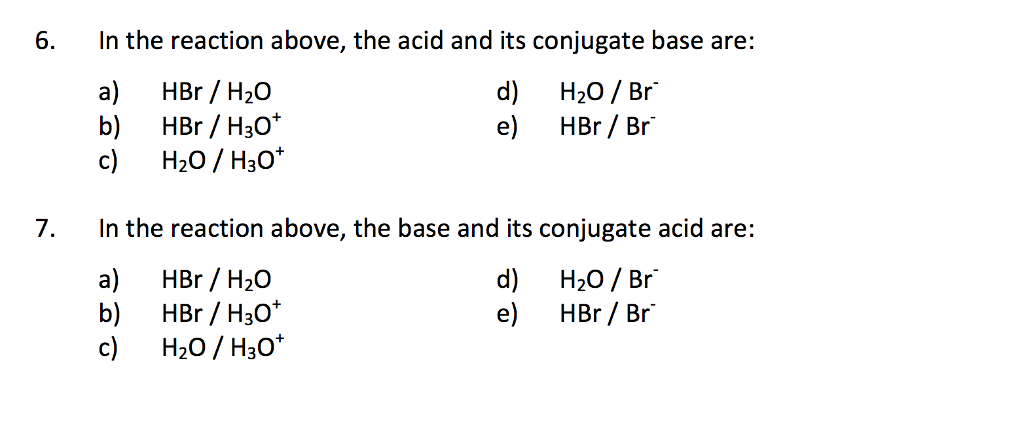
SOLVED: Identify the conjugate acid-base pairs in the reaction shown below HBr + NH3 Br + NH4 acid; chemPad Help Gianke conjugate base: chemPad Help Girekt base: chemPad Help Creke conjugate acid:
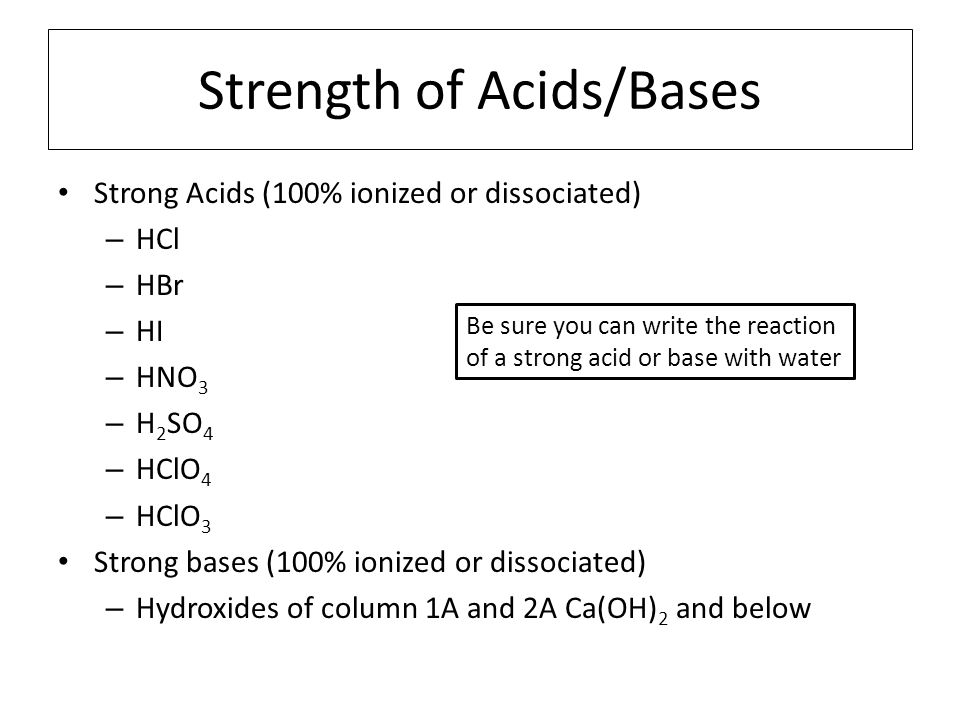
HL Acids and Bases. Strength of Acids/Bases Strong Acids (100% ionized or dissociated) – HCl – HBr – HI – HNO 3 – H 2 SO 4 – HClO 4 – HClO 3 Strong bases. - ppt download
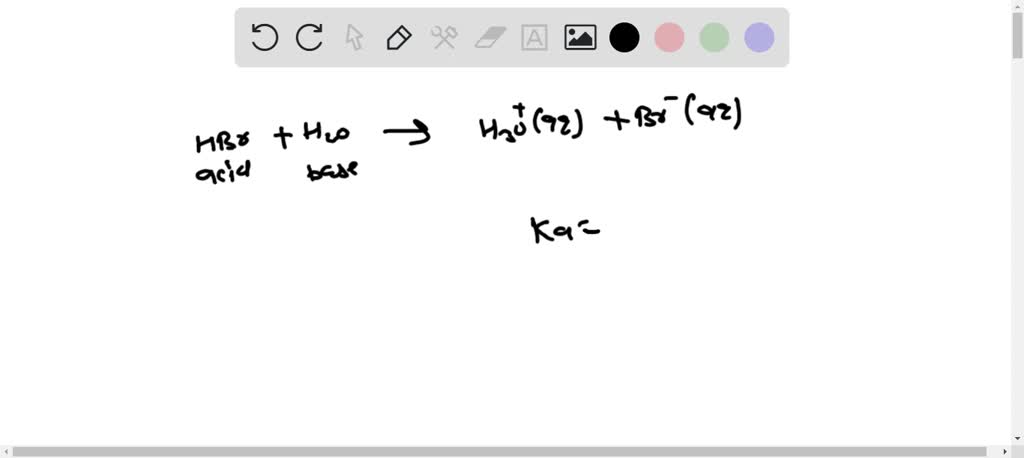
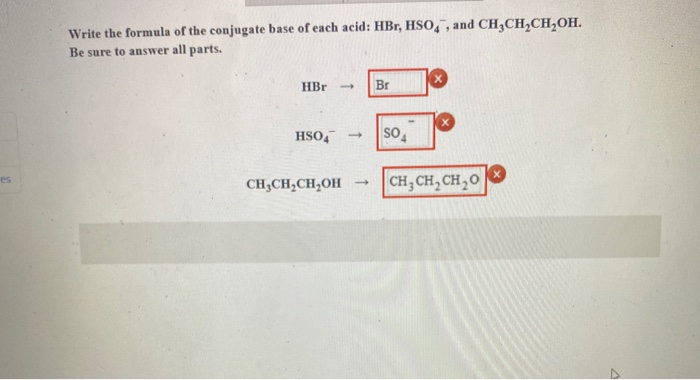
SOLVED: (a) write the net acid-base reaction that occurs when HBr is added to water. (use the lowest possible coefficients. Omit states-of-matter in your answer.)(b) What is the relative Ka value for