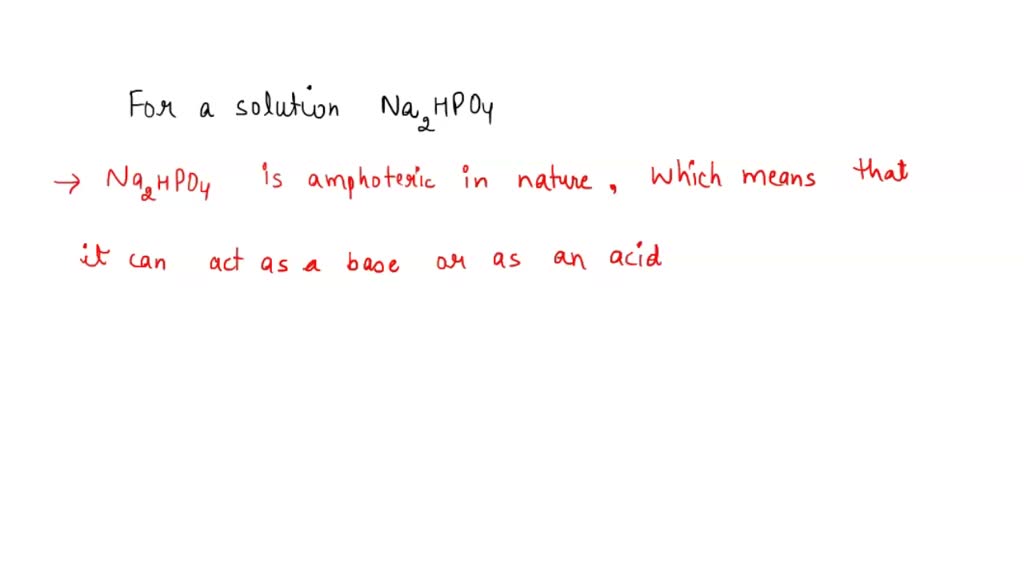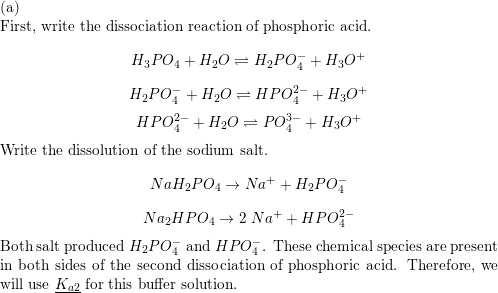Q. The equivalent mass of H3PO4 (Molecular weight = 98 g/mol) and Na2HPO4 (Molecular weight = 142 g/mol) in the reaction are respectively : H3PO4 + 2NaOH → Na2HPO4 + 2H2O (1) 49, 142 (2) 49, 71 (3) 98, 71 (4) 98, 142
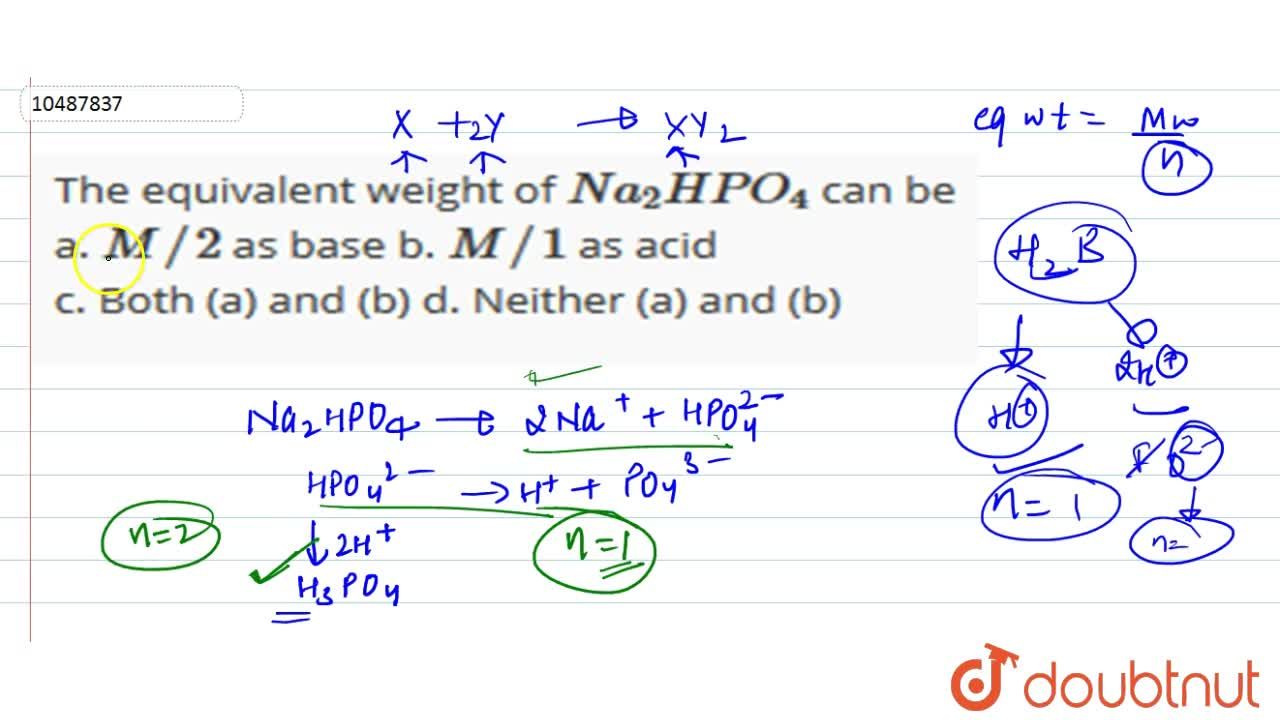
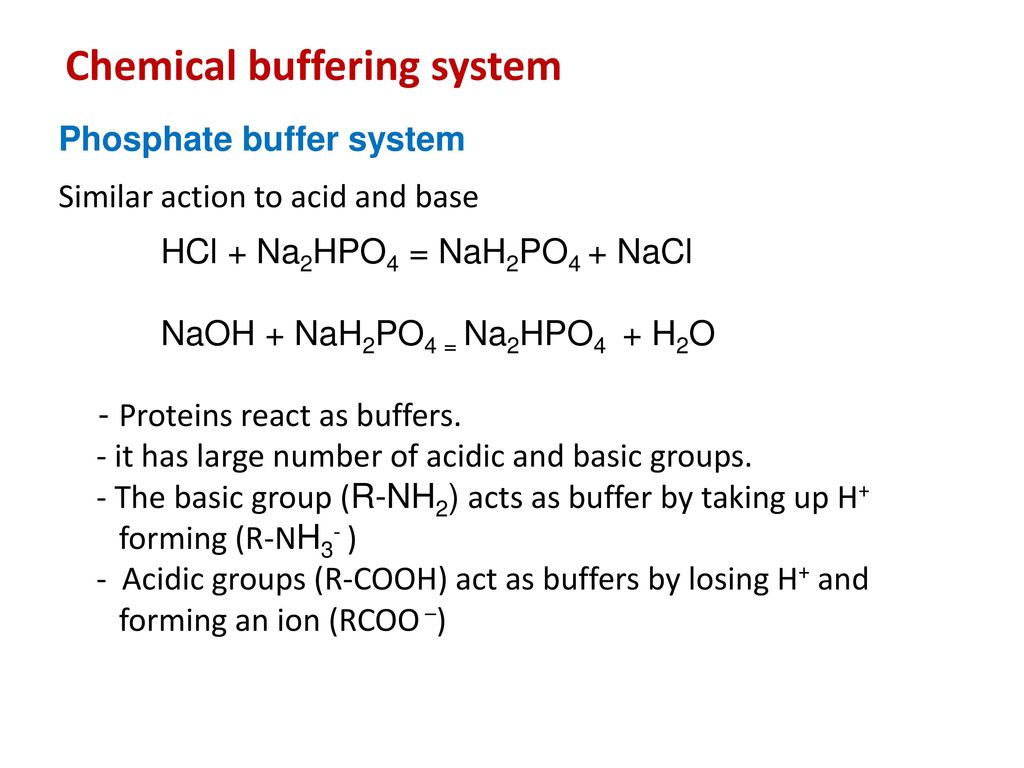
The equivalent weight of Na(2) HPO(4) can be a. M//2 as base b. M//1 as acid c. Both (a) and (b) d. Neither (a) and (b)

OneClass: Equal molar quantities of sodium hydroxide and sodium hydrogenphosphate (Na2HPO4) are mixed...

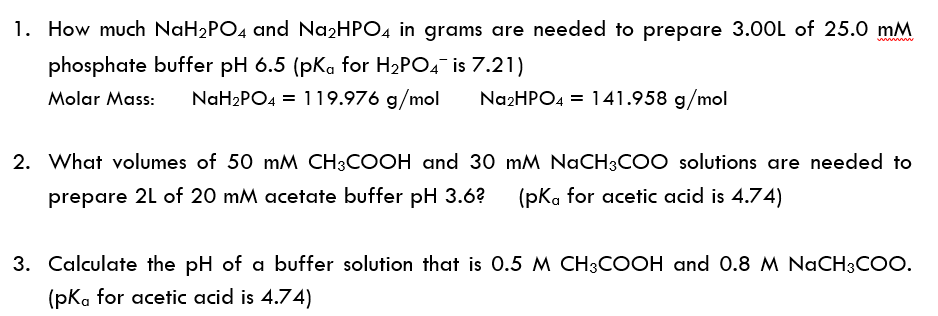
Calculate the pH of a buffer solution obtained by dissolving 25.0 g of KH2PO4(s) and 38.0 g of Na2HPO4(s) in water and then diluting to 1.00 L. | Homework.Study.com

A buffer solution 0.04 M in Na2HPO4 and 0.02 M in Na3PO4 is prepared. The electrolytic oxidation of 1.0 milli - mole of the organic compound RNHOH is carried out in 100
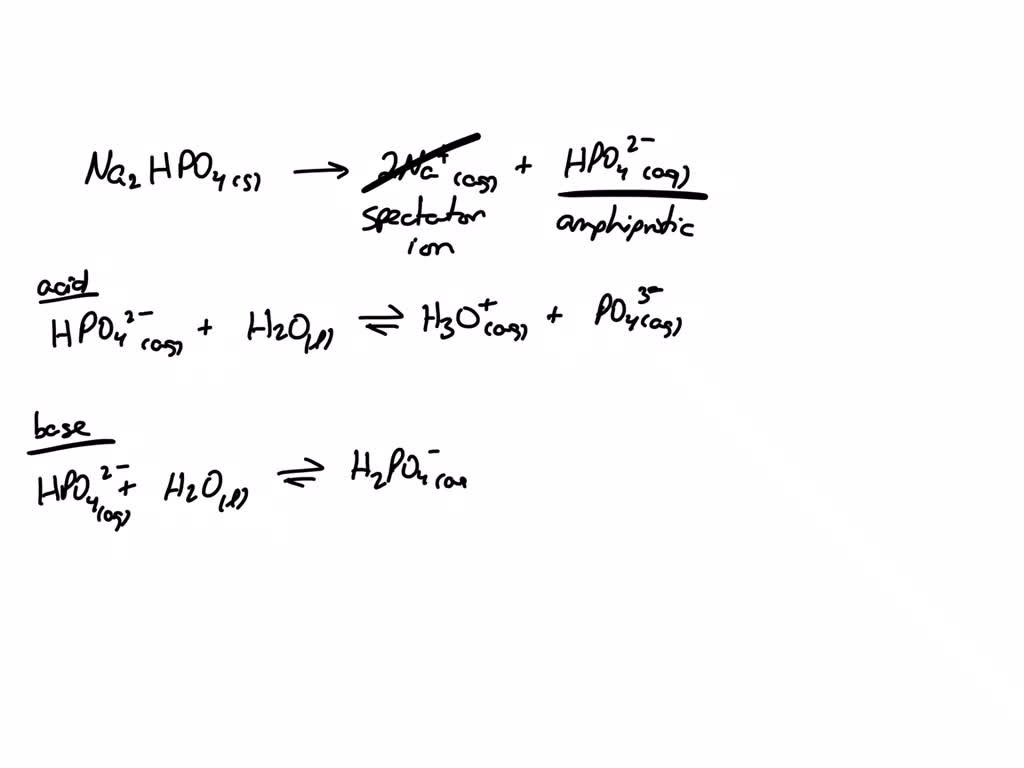
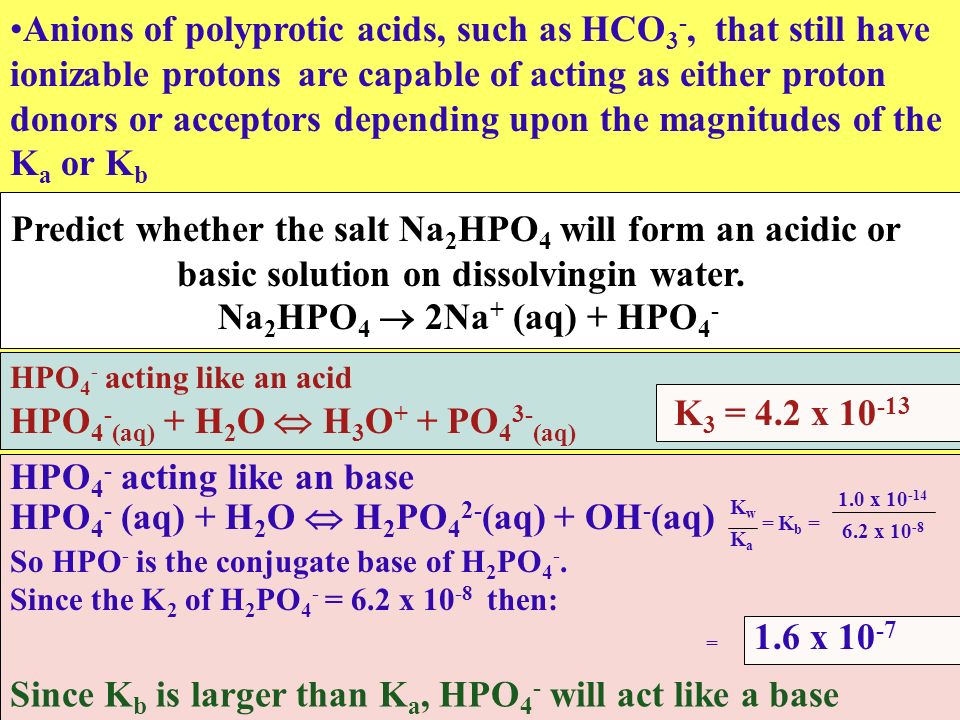
SOLVED: Predict whether the salt Na2HPO4 forms an acidic solution or a basic solution when dissolved in water.






![An example of an acid salt is [CH(3)COONa//NaNO(3)//Na(2)HPO(4)//NaKCO(3)] An example of an acid salt is [CH(3)COONa//NaNO(3)//Na(2)HPO(4)//NaKCO(3)]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/643651395_web.png)