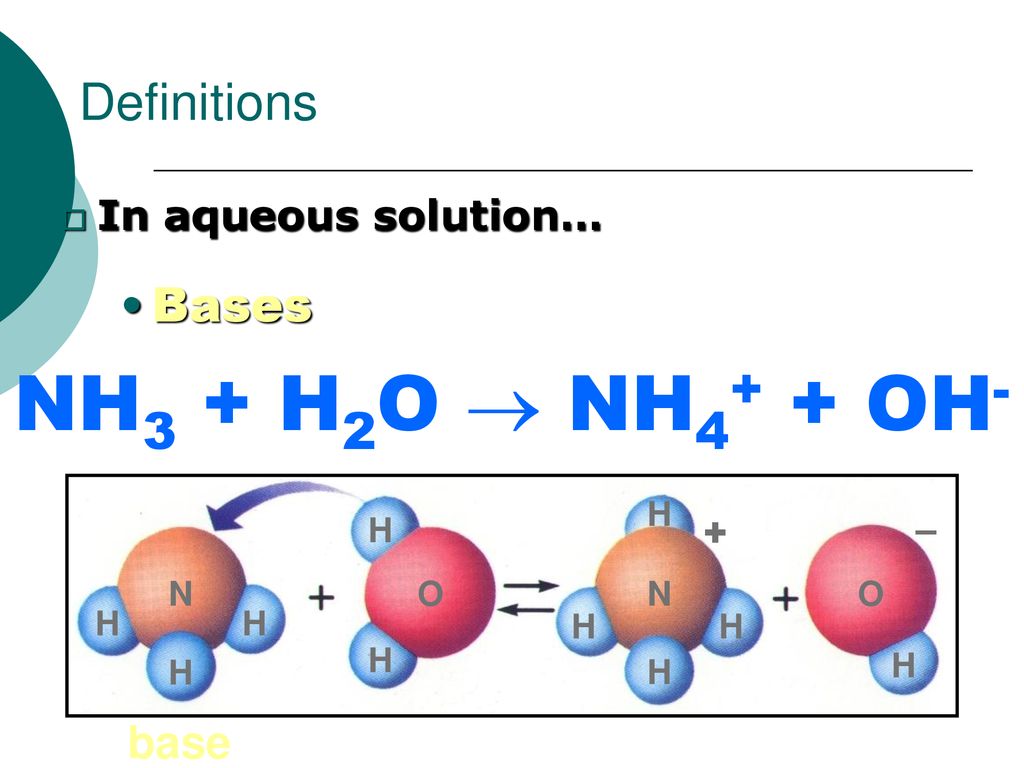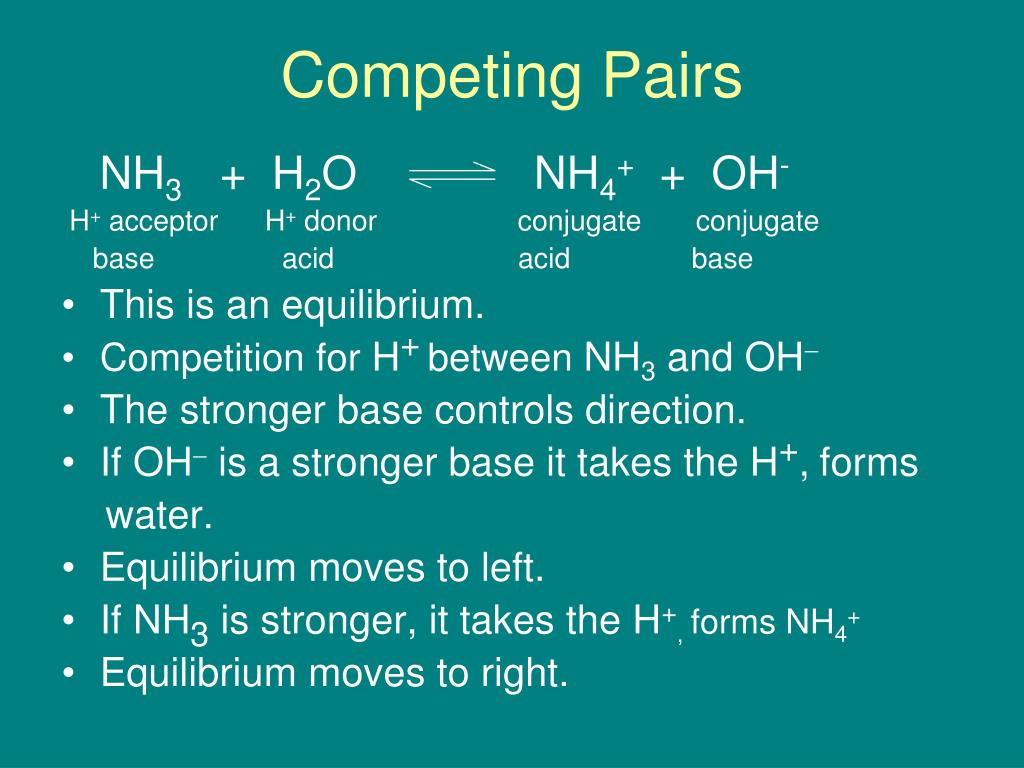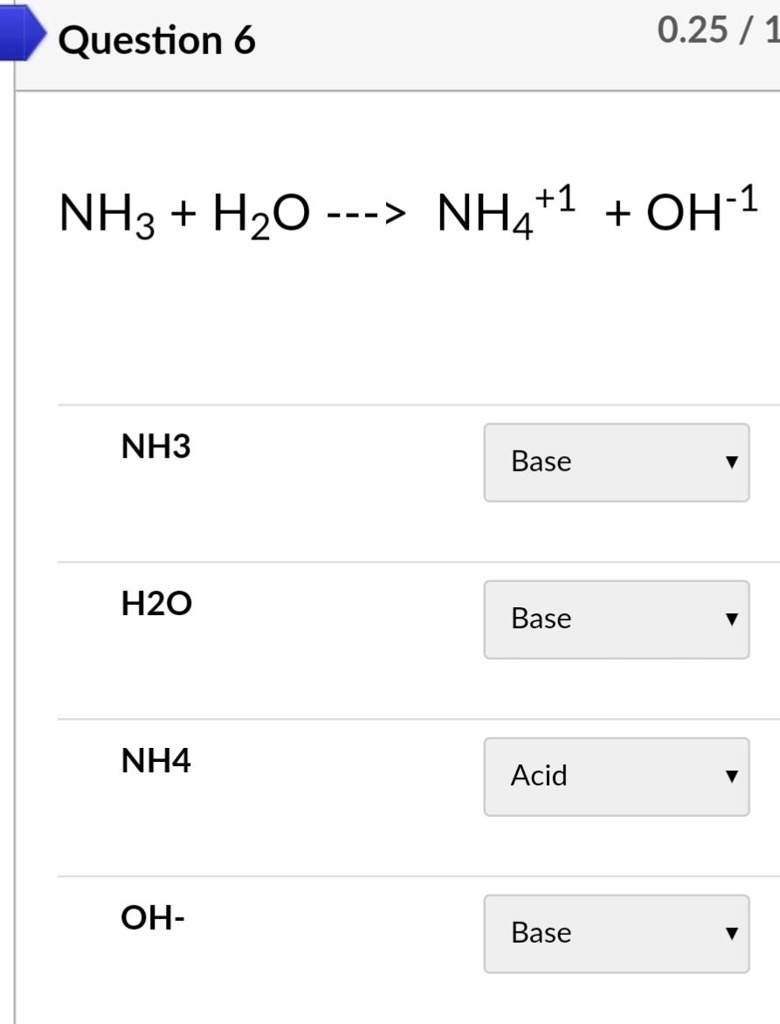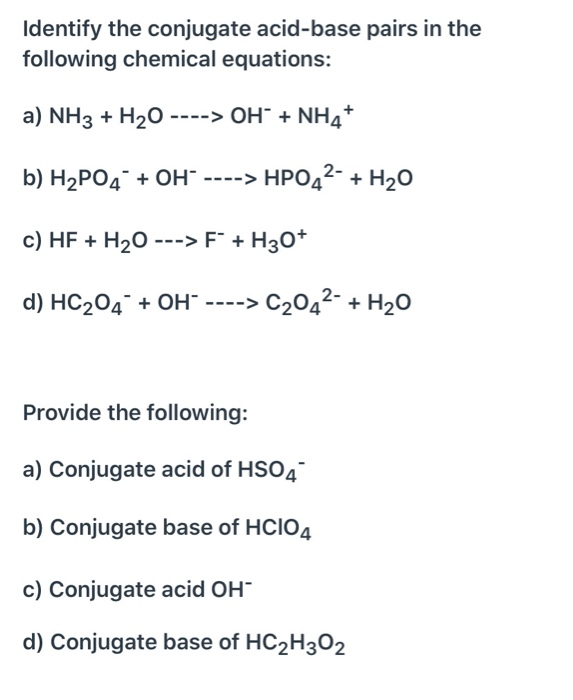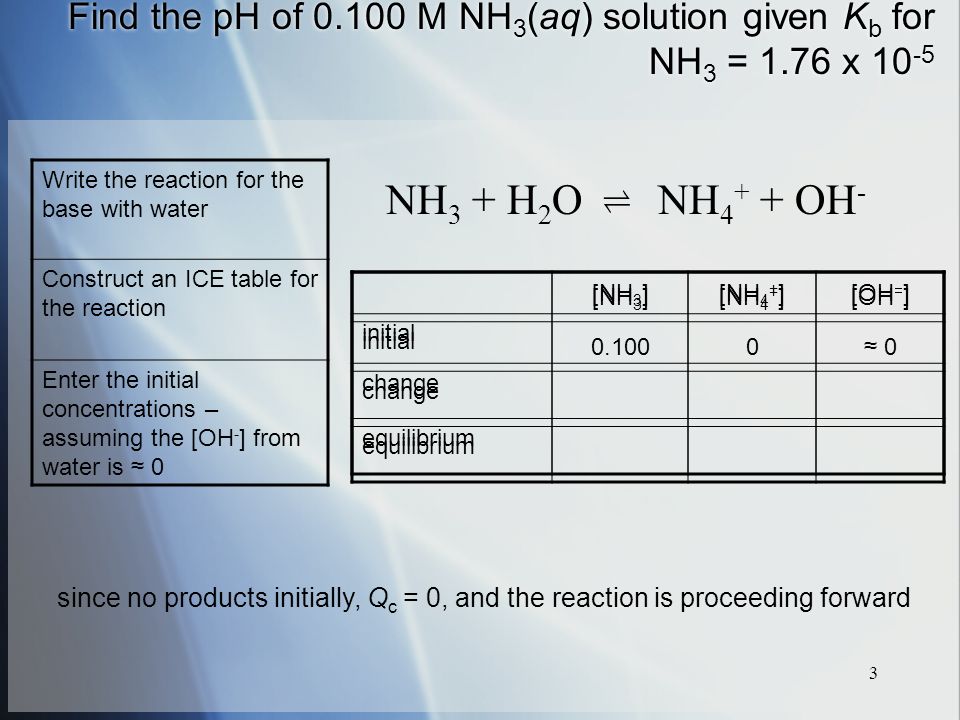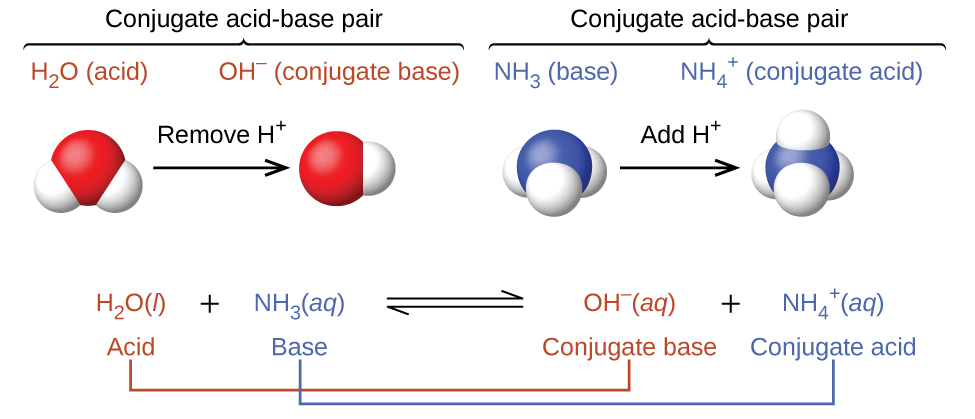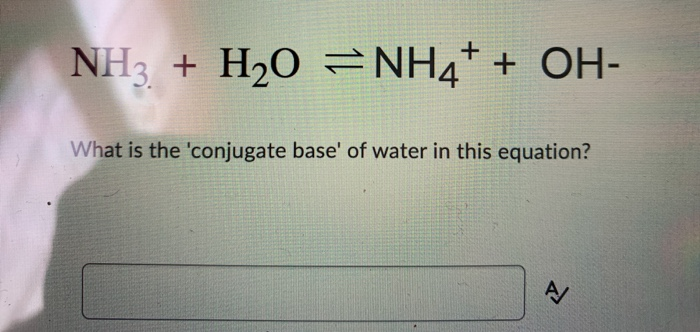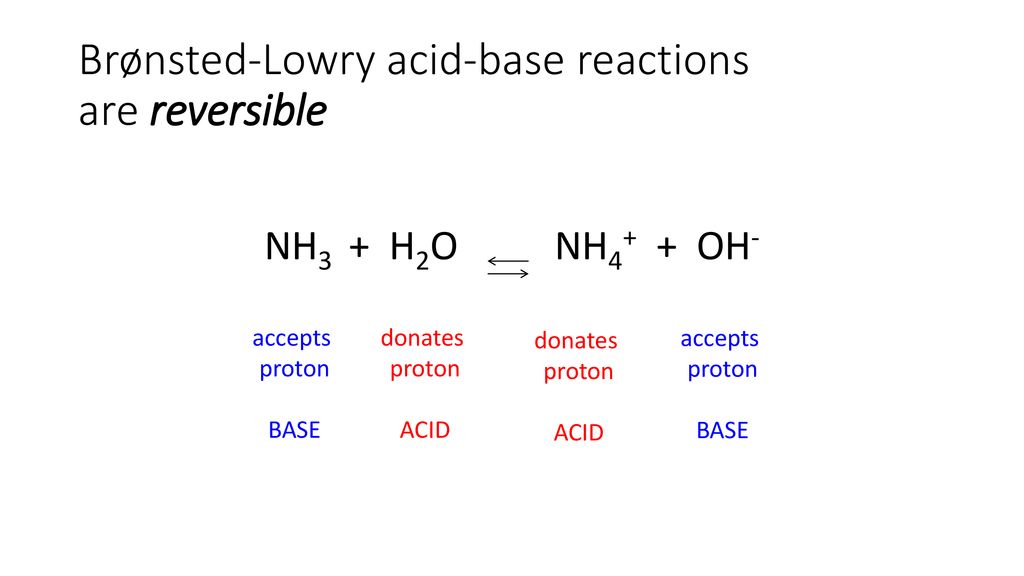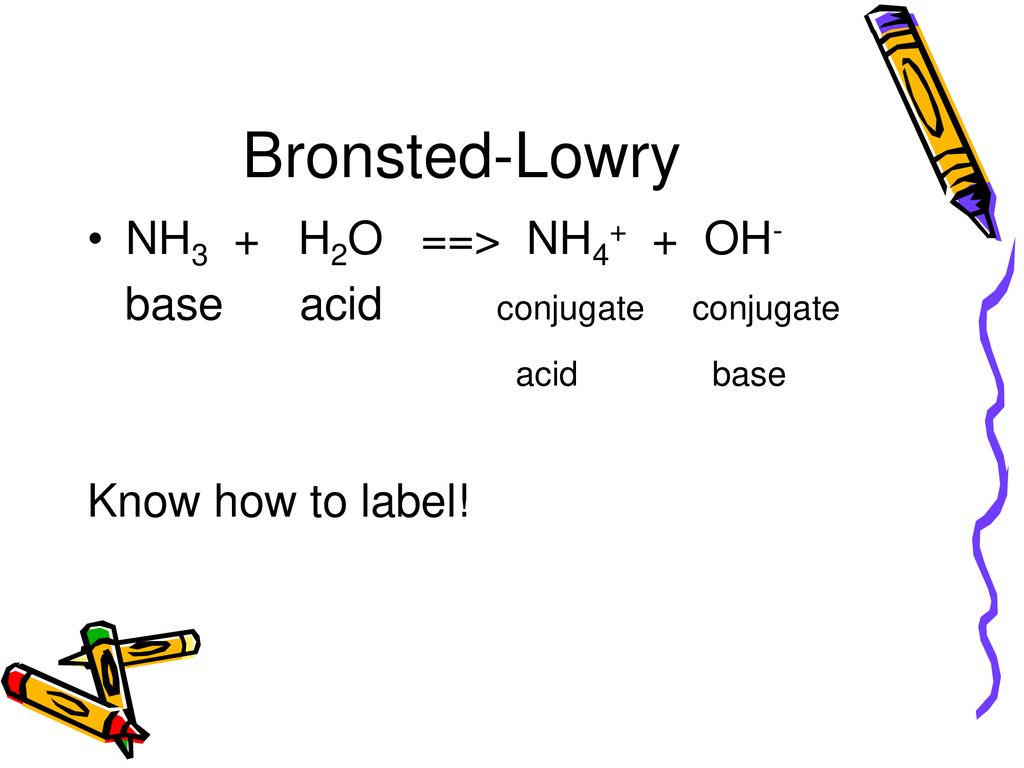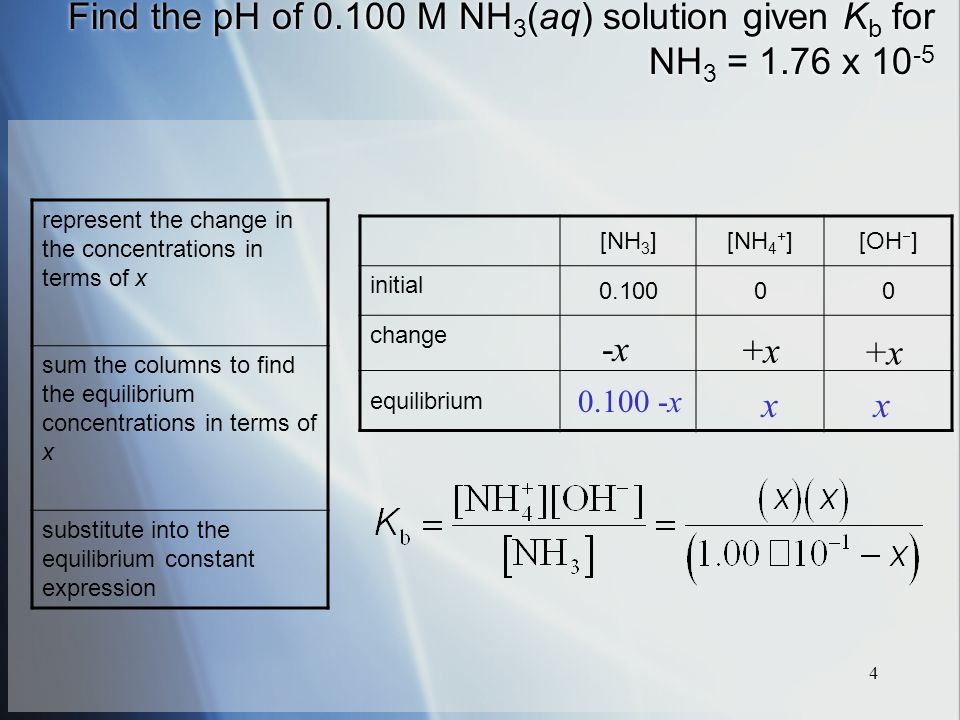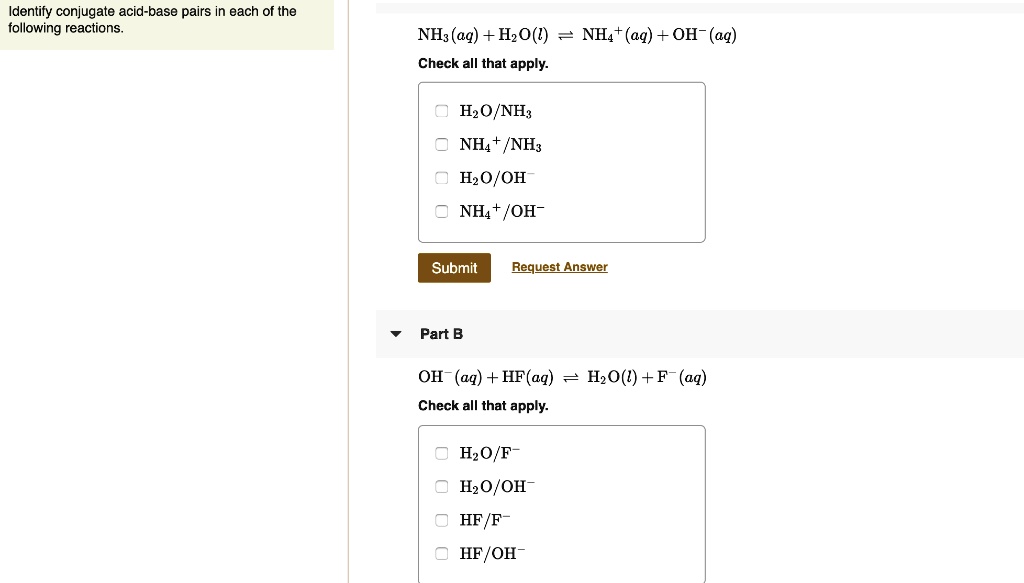
SOLVED: Identify conjugate acid-base pairs in each of the following reactions NH; (aq) + HzO() Check all that apply: NH4 + (aq) + OH- (aq) HzO/NH: NH4 NHz HzO/OH NH4 + /OH-
Solved] Consider the reaction below: NH3 + H20 --> NH4* + OH Who is the conjugate acid in the forward reaction? | Course Hero