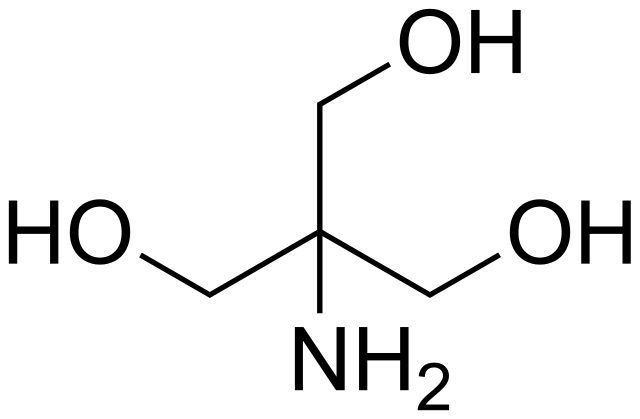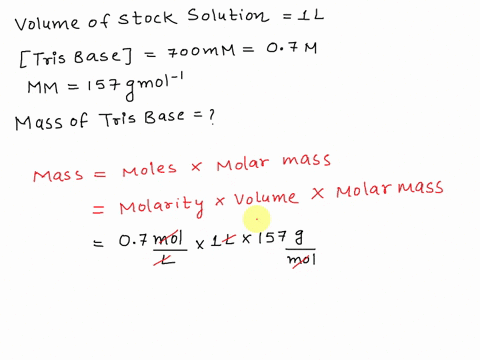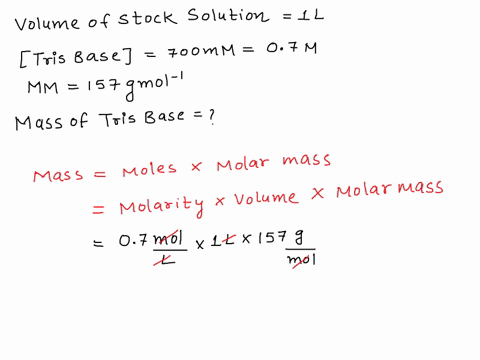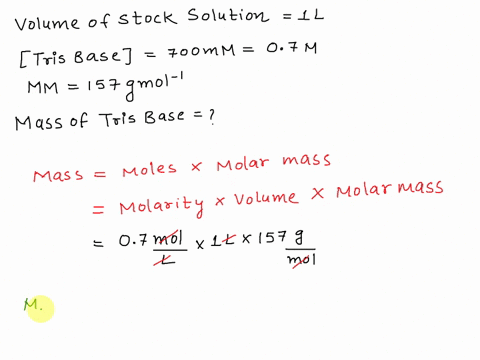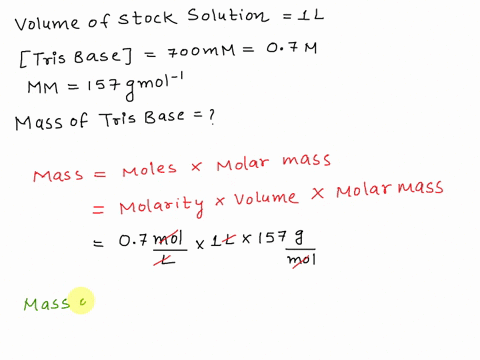
SOLVED: The composition of 1X TBE is: 89 mM Tris (molar mass = 121.14 g/mol), 89 mM Boric Acid (molar mass = 61.83 g/mol), 2 mM EDTA (molar mass = 292.24 g/mol).
STOCK SOLUTION RECIPIES: Tris-HCl Buffer 10X Tris-HCl (0.5M Tris Base, pH7.6): Trizma Base ---------------------------------- 6
TRIS, 500 g, CAS No. 77-86-1 | null | Buffer Solutions and -Salts | Reagents for Histology | Histology/Microscopy | Life Science | Carl Roth - International
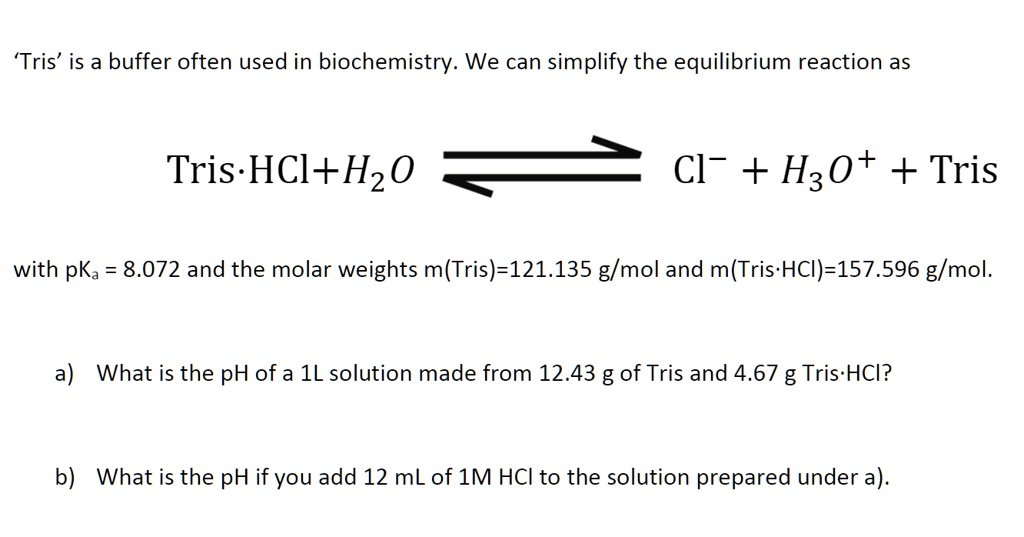
![Tris Hydrochloride [C4H11NO3.HCl] Molecular Weight Calculation - Laboratory Notes Tris Hydrochloride [C4H11NO3.HCl] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2022/11/tris-hydrochloride-molecular-weight-calculation-300x225.jpg)


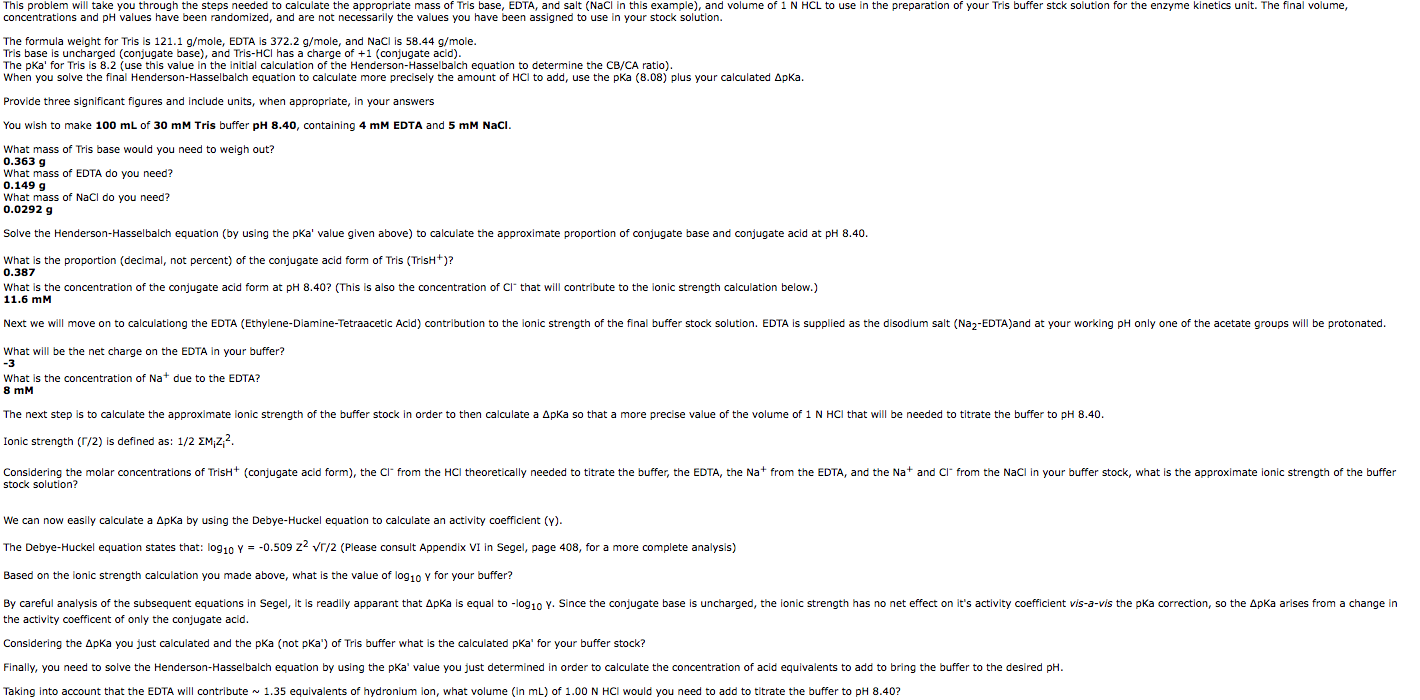


![T60040-500.0 - Tris Base Ultra Pure [Tris (Hydroxymethyl) Aminomethane], 500 Grams T60040-500.0 - Tris Base Ultra Pure [Tris (Hydroxymethyl) Aminomethane], 500 Grams](https://d2gdaxkudte5p.cloudfront.net/system/images/T60040-500.0_.jpg)